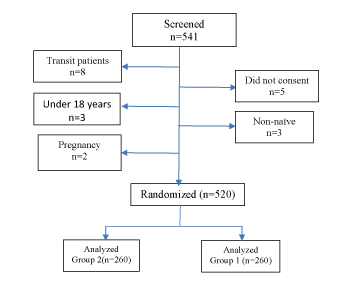
Figure 1:Study Patients Flow

Deus Buma1* Muhammad Bakar2 Wafaie Fawzi3 Ferdinand Mugusi2
1Department of Pharmacy, Muhimbili National Hospital (MNH), Dar es Salaam, Tanzania*Corresponding author: Deus Buma, Muhimbili National Hospital, P.O BOX 65000, Dar es Salaam, Tanzania, Tel: +255787 228282; E-mail: deus.buma@mnh.or.tz, buma70@yahoo.co.uk
Objectives: To compare the immunological and clinical effects of a further reduction of stavudine dose to 30 mg once-daily with that of a standard zidovudine containing antiretroviral therapy (ART) regimen in a prospective, open-label randomized controlled study.
Methods: Naïve HIV infected patients were equally randomized to receive either stavudine 30 mg once-daily or a standard dose zidovudine containing regimen. CD4+ T-cell counts, Haemoglobin (Hb), alanine aminotransferase (ALT), Body Mass Index (BMI), WHO stage and patients’ morbidity at baseline, three and six months were determined. Changes between baseline and three as well as six months follow-up were compared within-and between-groups.
Results: Five hundred and twenty patients aged ≥ 18 years were included. Males were 159 (30.6%), with the mean (SD) age of 39 (9) years. Within group comparison indicated that there were statistically significant increases in mean CD4+ cell counts, BMI, and Hb (p<0.0001) at 3 and 6 months from the values at baseline in both groups, but there were not significantly different between groups. Furthermore, there was no statistically significant difference between groups in terms of occurrence of opportunistic infections (OIs) however, there was significant decrease of OIs for subsequent follow up time points compared to baseline status. The overall median adherence rate was 94% (IQR 94%, 98%) in both groups, but patients in the stavudine group had better adherence compared to those in the zidovudine group, p<0.0001. Additionally, liver damage at 6 months, as indicated by elevated ALT, was more likely with the zidovudine based regimen, p <0.0001. There was no statistically significant difference in ALT elevations between the groups at 3 months.
Conclusion: The immunological and clinical outcomes following a regimen employing a reduced dose of stavudine to 30 mg once-daily were similar to those of a standard zidovudine-based antiretroviral regimen.
Stavudine; Zidovudine; CD4+cell counts; Adherence
Stavudine has been widely used for HIV treatment in resource-limited countries due to its low cost [1,2], favorable early tolerability, convenient drug administration, and minimal requirements for laboratory monitoring [3].
Stavudine inhibits mitochondrial DNA polymerase of adipocyte tissue, heart, nerve, liver and pancreatic cells [4], and this inhibition causes cumulative toxicity resulting in lipodystrophy (known as HIV lipodystrophy) especially lipoatrophy [5]. Beside lipodystrophy, peripheral neuropathy (PNP) and lactic acidosis are mostly encountered by patients on stavudine based regimen [6] while the other stavudine related adverse effects include cardiomyopathy, headache, rashes and pancreatitis [1,7].
Currently stavudine is no longer recommended by World Health Organization (WHO) for first-line antiretroviral regimens due to toxicity concerns [7-9]. While there is concern about stavudine related toxicity, some low income countries are still using stavudine based regimens among their combinations, simply because of budgetary constraints [2,10]. Complete phasing out of stavudine based regimens in countries like Tanzania which has serious budgetary constraints will likely limit access to antiretroviral therapy, and therefore may raise HIV infection incidence and HIV/AIDS related deaths. A change from stavudine based regimens to other regimens has about 50% or more increase in the cost per person per annum [11], and in some countries phasing out of stavudine has not been implemented completely [8,10].
Undoubtedly, all medicines especially antiretroviral drugs have side effects. Stavudine has tolerance among patients using it at lower doses [12]. The merits of stavudine are substantial to the extent that it may remain one of the alternative medicine when tenofovir, zidovudine and abacavir are not recommended, particularly among HIV infected patients with renal and hemoglobin abnormalities [13].
Studies indicate that reduction of stavudine dose from 40 mg (80 mg per day) to 30 mg (60 mg per day) twice-daily was associated with a significant reduction of stavudine related toxicity [3,14,15]. A study by Pujades-Rodriguez in 2011 [3] indicated that higher rates of drug-related toxicity were reported in patients receiving stavudine 40 mg (80 mg per day) compared with 30 mg (60 mg per day), and the time to toxicity diagnosis was shorter in patients treated with the higher dose. Further reduction of stavudine dose from 40 mg (80 mg in a day) to 20 (40 mg in a day) as well as from 30 mg (60 mg in a day) to 15 mg (30 mg in a day) indicated significant further reductions of stavudine related metabolic abnormalities [12,16].
The current stavudine label suggests that toxicity can be reduced by administering even lower doses but given twice daily, that is 60 mg in a day. In all studies so far, the effectiveness of once-daily administration of 30 mg stavudine in combination with other antiretroviral drugs against HIV infected individuals is lacking. It is unclear if administration of the drug as a once-daily reduced dose will have similar outcomes as standard regimens. To address this question, we assessed the non-inferiority of stavudine based regimen at aforementioned low dose of 30 mg compared to a standard dose zidovudine (AZT)-based regimen on immunological and clinical outcomes at 24 weeks. Stavudine was compared to zidovudine rather than tenofovir because, during the study period zidovudine was the default drug in the initiation of antiretroviral therapy (ART) in Tanzania.
This was an open-label, randomized, and controlled study, in which clinicians, pharmacists and patients were aware of the study regimens provided. However, allocation of patients to a particular study group was randomly generated by a randomization software developed by Saghaei [17].While the software did a random allocation of 520 patients in a ratio of 1:1, block randomization was done in the blocks of twentieths, each block maintained the ration of 1:1. Regimen numbers in each block were printed and put in the opaque envelope, mixed up in a box, and then numbered from one through 20. Patients were assigned the study regimens on subsequent basis in the form of first-in-first allocated.
HIV-infected subjects (aged ≥ 18 years) were recruited from Care and Treatment Clinics (CTC) supported by Management and Development for Health (MDH) program in Dar es Salaam region. The MDH program is supported by US President’s Emergency Plan for AIDS Relief (PEPFAR) to support HIV clinics within Dar es Salaam city. The clinics are located within the hospitals or health centers and include Muhimbili National Hospital (MNH); Temeke, Amana, and Mwananyamala hospitals; Infectious Disease clinic (IDC); and Mbagala, Sinza, Mnazi Mmoja, Buguruni and Tabata health centers. The clinics have enrolled about 70,000 patients and about two-thirds of them are women. Among these patients, about 7,000 were on care and monitoring at Mwananyamala, Amana, IDC and Temeke hospitals as they were not eligible for antiretroviral therapy. About 40 patients were identified on a daily basis as eligible and thus initiated on antiretroviral therapy. The study participants were recruited from four CTC’s which were at the IDC, Amana, Temeke and Mwananyamala hospitals. Selection of the study sites was based on the high enrolment and antiretroviral therapy initiation rates of eligible HIV infected patients.
Inclusion criteria required that patients be HIV-infected as confirmed by positive HIV antibody test, able to give informed consent, and eligible for initiation of antiretroviral therapy and naïve to antiretroviral medicines as determined by self-reporting. Exclusion criteria included pregnant women and women who indicated intention to become pregnant during the study period as determined during initial assessment, a history of mental disorder, clinically diagnosed peripheral neuropathy and lipodystrophy.
Five hundred and twenty subjects were randomized equally to one of the two regimens according to group randomization software method established by Saghaei [17]. The sample size was calculated based on 90% power, at significance level of 5%, assuming no differences between the two groups in a two sided study. Patients in group one received a standard regimen that contained zidovudine (AZT) at 300 mg, lamivudine 150 mg given twice daily and efavirenz at 600 mg, while those in group two received a modified regimen that contained stavudine 30 mg, lamivudine 300 mg and efavirenz 600 mg, all given as once-daily doses [18]. The study drugs were provided free of charge by the Tanzanian Government through the Medical Stores Department (MSD). Patients visited the clinic on a monthly basis with a total follow up period of six months (24 weeks). Patients were asked to remain on the assigned medication unless these were changed by the study clinician based on presence of untoward effects.
Recruitment of patients started on November 7th, 2011. Patients were followed up for six months. At enrolment demographic information and anthropometric data were recorded. At baseline and on a monthly basis, clinical assessments were conducted and recorded on a case report form (CRF). We diagnosed lipodystrophy by use of a standardized case definition-based questionnaire and clinical assessment at baseline and six months. This instrument has been validated in African settings and is a combination of patient self-assessment and inspection by the clinician of body areas (face, neck, chest, abdomen, arms, legs and buttocks). Body shape changes were scored as absent, mild, moderate or severe [9,12]. We assessed peripheral neuropathy (PN) based on clinical methods in which sensory examination was used (joint position, touch, vibration and pain sensation).
Medicines were provided on a monthly basis, and adherence was assessed each month and the average was obtained, based on the two methods; pill count (PC) and self-reported (SR) [19]. Patients were asked to return back with their medicine containers for refill or clinical assessment at each visit. The dispenser counted the remaining tablets before dispensing medicines for the following month. Also the dispenser asked patients whether or not they had missed doses for the past thirty days. Adherence was expressed as a percentage based on the number of pills taken out of the monthly supply, as well as deducting the number of missed days from the total number of days a patient was supposed to take. If the patient was taking twice-a-day dosing, each dosing was considered as half a day dosing.
Hemoglobin was measured at baseline, three and six months by Pentra 80 (ABX Pentra 80 serial number 602P802149, France), while liver damage was determined by serum alanine aminotransferase (ALT) measured at similar intervals by Cobas Integra 400 Plus (Roche Diagnostic Ltd SN399096 Rotkreuz, Switzerland). CD4+ T lymphocyte (CD4 count) was measured at baseline, three and six months by FacsCalibur (BD Biosciences serial number E9750071, USA).
The primary outcomes were changes in CD4+ cell counts, the development of stavudine associated side effects (peripheral neuropathy and lipodystrophy) and the occurrence of Opportunistic Infections (OI’s).
Data were double entered into a secure Microsoft Access database. For statistical analyses Stata for windows software (version IC/12.1; 4905; Stata corp; College Station, Texas 77845 USA) was used. The intention-totreat method was used during analysis because there was no lost to follow up, change of regimens or death. Patients’ baseline characteristics were compared and statistical tests were performed using Chi-squared and t-tests for categorical and continuous variables respectively. The prevalence of clinical features at enrolment and their incidences during follow-up were expressed with 95% confidence intervals. At six months, cumulative incidences of lipodystrophy with the relative occurrence between the two regimens were examined, including 95% confidence intervals. Between group comparison of CD4+ cell counts and haemoglobin were performed using student t-test. A paired t-test was used to assess changes in haemoglobin and CD4+ cell counts at baseline, and at three and six months as well as between baseline and three and six months time points. All tests were 2-sided and were tested at 5% level of significance.
We classified age groups as 18-25 years, 26-30years, 31-40years and equal or above 41 years. We classified CD4+ cell counts into less than 350 and equal or above 350 cells/µl. Haemoglobin content was classified as normal if it was equal or greater than 12 g/dl and 13 g/dl for female and male respectively, anemia if it was less than 12 g/dl and 13 g/dl for female and male respectively. Marital status was classified as single if patients were unmarried, separated or widowed, while couples were considered if they were married or co-habiting.
Education level was classified as level one, two or three according to the level of education attained. Level one included patients who did not attend any formal education and primary education, level two included patients who attended either one, two or both of ordinary secondary school, advanced secondary school and vocational training, while level three were those who attained diploma and degree levels of education. Nutritional status was categorized based on the body mass index (BMI) as Underweight (less than 18.5 kg/m2), normal weight (18.5-24.9 kg/ m2 ), overweight (25.0-29.9 kg/m2 ) and obese (equal or above 30 kg/m2 ). Lipodystrophy was classified as absent (if there was no fat loss or gain) and present (if there was fat loss or gain regardless of severity). Liver function status was classified as normal if serum ALT level was less or equal to 40 U/L and abnormal if ALT level was above 40 U/L. We classified adherence as poor if attained less than 91% (missed doses more than three days in a month), good if attained between 91% and 94% (missed doses between two and three days in a month) and excellent if patients attained greater or equal to 95% (missed doses for at most one and half days).
The institutional review board (IRB) of Muhimbili University of Health and Allied Sciences (MUHAS) approved the study. Permission was granted by the respective Municipal authorities where the facility resided. The study was registered by the Pan African Clinical Trial Registry (PACTR) with the registration number PACTR201208000402280. Patients provided written informed consent prior to enrollment.
A total of 520 patients (260 in each group) were included in the analysis from the 541 screened as shown in Figure 1. Among them, 159 (30.6%) were males, and 361 (69.4%) were females. The overall mean (SD) age was 39 (9) years, while it was 41 (9) and 38 (9) years for males and females respectively. There was no loss to follow up, change of regimen or death during the study period.
In terms of social-demographic characteristics, 285 (54.8%) patients were living with a sexual partner (married or co-habiting), while 235 (45.2%) were living alone (single, separated or widow). The proportions with level one, two and three of education were 84.8%, 13.5% and 1.7% of participants respectively. As shown in Table 1, baseline socio-demographic characteristics were similar between the two treatment groups.
The two treatment groups were similar at baseline in terms of mean BMI, WHO clinical stage distribution, mean haemoglobin, mean serum ALT levels, liver status, as well as mean and distribution of CD4+ cell counts. However, BMI distribution revealed a higher proportion of overweight individuals among the stavudine group, p=0.007 (Table1), this happened by chance. No significant differences in the occurrence of opportunistic infections between the sexes at the initiation of therapy were found, and there were no statistically significant differences between the groups in terms of distribution of OIs.
Occurrence of OI’s : Overall, there was a decline over time in the prevalence of OIs, being 17.5% at baseline and then dropping to 7.3% and 4.0% at three and six months respectively (Figure 2). No differences were noted in the rate of developing OI’s between the two groups at both three and six months of follow up, The most common conditions reported by clinician were oral candidiasis (4.5%), tineacorporis (14.6%), impetigo (11.2%) and urinary tract infection (9.0%).

Figure 1:Study Patients Flow
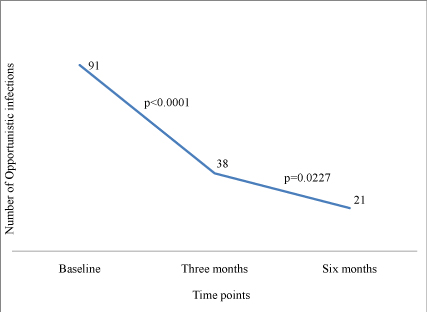
Figure 2:Change in the number of Opportunistic Infections over time
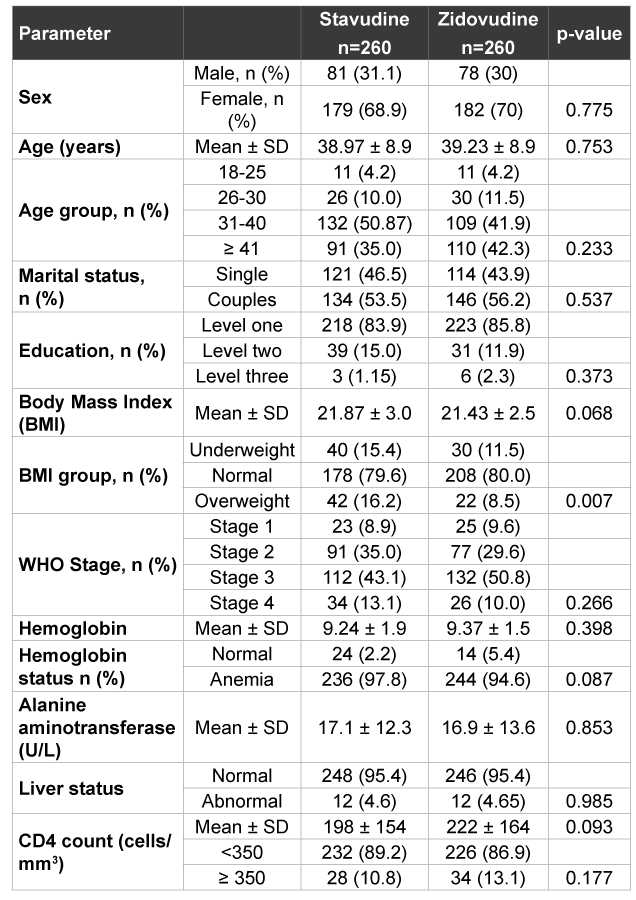
Table 1: Baseline characteristics of study patients
Hematological and other laboratory abnormalities: The mean Haemoglobin values at both three and six months of follow up were significantly higher in the stavudine group compared to those in the zidovudine group. Whereas the Mean (SD) Haemoglobin value at 3 months was 10.3 g/dl for the stavudine group, the value was 10.1 g/dl for the zidovudine group, p= 0.0476. On the other hand, the Mean (SD) Haemoglobin value at 6 months was 11.4 g/dl for the stavudine group, while the value was 9.7 g/dl for the zidovudine group, p<0.0001.
The mean serum ALT levels at 3 months were similar in the stavudine and zidovudine groups, being 19.7 U/L and 21.6 U/L, respectively, p=0.1227. However, at 6 months of follow up, patients in the zidovudine group had a higher mean serum ALT level (of 27.9 U/L) compared to those in the stavudine group (21.2 U/L), p<0.0001.
The proportions of patients with hepatotoxicity (defined as serum ALT ≥ 40 U/L) by sex and regimens at three months showed no statistically significant differences.
At six months of follow up the proportions with hepatotoxicity were also not statistically significant between the sexes (being 20.4 among males, and 13.9% among females, p=0.065. However, the proportion with hepatotoxicity among females in the stavudine group of 10, 1% was significantly lower compared to that of 17.7% among females in the zidovudine group, p<0.0384.
Drug related adverse effects: Overall, there was a significant decrease in the cumulative number of ARV’s-related complications at three and six months of follow up (Figure 3). The frequencies of (16 at 3 months, 7 at 6 months) of reported drug related adverse effects among patients in the stavudine group was lower than the frequencies of (63 at 3 months, 9 at 6 months) among patients in the zidovudine group, p=0.0454.
During follow up there was only one case with features suggestive of lipodystrophy among patients in the zidovudine group. There was no one in the stavudine group.
As shown in Figure 4, although there was an overall significant rise in CD4+ T-cell counts (p<0.0001) in both study groups when compared with their baseline reference values. While in the stavudine group the mean (SD) count at baseline, 3 and 6 months were198 (153), 268 (179) and 320 (190) cells/ml respectively, the values for the zidovudine group were 222 (164), 288 (164) and 330 (158) cells/ml respectively. It should be noted that there were no statistically significant differences between the once-daily stavudine based regimen and the zidovudine based standard regimen in terms of mean CD4+ T-cell counts achieved at 3 and 6 months periods.
When patients were stratified by sex and regimen it was found that at 3 months of follow up the proportion of males with CD4+ T-cell counts less than 350 cells/mm3 was 76.5% in the stavudine group compared to 79.2% in the zidovudine group (ie a difference of 2.7%), p= 0.6831. On the other hand, the proportion of females with CD4+ cell counts less than 350 cells/mm3 was 78.1% in the stavudine group compared to 71.8% in the zidovudine group (ie. a difference of 6.3%), p= 0.2327. While, at 6 months of follow up the proportion of males with CD4+ T-cell counts less than 350 cells/mm3 was 64.2% in the stavudine group compared to 67.5% in the zidovudine group (ie a difference of 3.3%), p= 0.7227. On the other hand, the proportion of females with CD4+ cell countsless than 350 cells/mm3 was 69.1% in the stavudine group compared to 67.0% in the zidovudine group (ie. a difference of 2.1%), p= 0.7245.
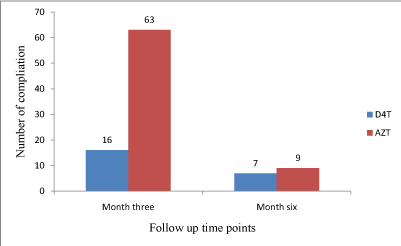
Figure 3: Number of ARVs complications over time
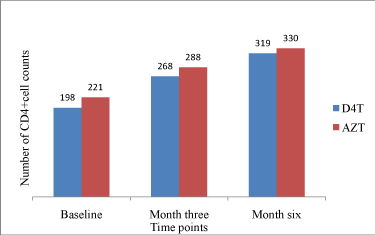
Figure 4: Number of CD4+ cell counts (cells/mm3) over time
The overall adherence to prescribed ARV’s was 94%. This was distributed as being excellent (46.6%); good (48.8%); and poor (6%) adherence among the 520 study patients.
A statistically significant difference between the groups at six months was found in adherence to ARV’s, p<0.0001. Patients in the zidovudine group were more likely to have missed doses in a month compared to those in the stavudine group, with a risk ratio (95% CI) being 1.152 (1.099, 1.209).
No statistically significant differences in adherence to treatment were noted between the sexes or age groups, p = 0.908 and 0.066 respectively.
At three months of follow up, patients with CD4+ cell counts ≤ 350 cells/ mm3 at baseline were more likely to adhere to ART treatment compared to patients with higher baseline CD4+ T-cell counts, with a risk ratio (95% CI) being 0.4 (0.210, 1.760; p=0.0045). Similarly, at the sixth month of follow up, patients with CD4+cell counts ≤ 350 cells/mm3 at baseline were more likely to adhere to ART treatment compared to patients with higher baseline CD4+ T-cell counts, with a risk ratio (95% CI) being 0.554 (0.290, 1.060; p=0.0715).
To our knowledge, this is the first randomized, controlled trial to assess the clinical and immunological effects of an ART regimen with stavudine 30 mg given once-daily compared to the standard regimen (zidovudine based) in a developing country setting. Also our study was the first whereby patients were given once-daily stavudine (30 mg in 24 hours) instead of twice daily (60 mg in 24 hours) to HIV infected patients eligible for therapy. Stavudine has long intracellular half-life; its mechanism of action is based on viral DNA termination as nucleotide analogue after phosphorylation, therefore warrants once-daily use in combination with other antiretroviral medicines.
In this study we did not find the differences in terms of occurrences of opportunistic infections between stavudine and zidovudine groups. Our findings indicated that patients with CD4+ cell count less than 350 cells/ mm3 had more opportunistic infections than those with higher readings. Similar findings were observed by studies conducted by Hoffman et al., William et al., Kranzer et al. and Hogg et al. [20-23].
Our study showed that patients on the stavudine group had higher haemoglobin level compared to those in zidovudine group at the end of follow up. Similar findings were observed in other studies whereby zidovudine was found to be significantly associated with a decrease in haemoglobin level compared to comparator drugs [24,25]. Also our findings did not differ from other studies, which showed that zidovudine was associated with anemia [26-29].
It has been shown in this study that liver damage (as determined by a rise in serum ALT) increased significantly from baseline to the subsequent follow up time points in both groups. Similar findings were reported by Kovari et al. in 2010 on the elevation of serum ALT by both stavudine and zidovudine [30]. Our findings indicated that stavudine was less likely to be hepatotoxic more at six months follow up. Our findings are similar to those reported by Monforte Ade et al. [31] who reported a low frequency of severe hepatotoxicity in the stavudine based regimen compared to zidovudine based regimen in HIV-positive patients treated with HAART.
In this study we did not find the differences in terms of adverse effects (AEs) between stavudine and zidovudine groups. Our study did not report the occurrence of peripheral neuropathy at six months, probably due to a short follow up period. However, lipodystrophy was reported in one patient on zidovudine group [32] but not on stavudine group. The occurrence of lipodystrophy in such a short period of time can probably be augmented by the fact that HIV infection per se is associated with lipodystrophy as reported by Fuller [33].
It was found in this study that use of stavudine based regimen resulted in statistically significant increases in CD4+ cell count after a 3 or 6 months period. This is in keeping with findings reported by Zhou et al. [34] who reported significant increase of CD4+ cell counts from baseline to subsequent follow up time points using stavudine in combination with nevirapine and didanosine at the standard doses.
Importantly, this study showed that there were no statistically significant differences between a regimen employing stavudine (30 mg in a day) and a regimen employing zidovudine (standard dose) in terms of CD4+ cell counts at the follow up time points [35]. Our study also indicated that patients with mean baseline CD4+ cell counts of less than 350 cells/mm3 had lower CD4+ cell counts compared with those with above which at six months of follow up. Similar findings were observed in the study conducted at Peruvian National Program that investigated CD4+ cell recovery and adverse outcome after highly active antiretroviral therapy (HAART) which indicated that the mean increase in CD4+ cell counts at six months was 114 cells/microL (95% confidence interval: 103-126). Patients with a lower CD4+ cell counts at baseline had a lower increase in CD4+ cell counts at six months [36].
Our findings indicated that about 47% of patients had more than or equal to 95% adherence to treatment for the entire follow up period. Simplification of treatment regimen to once daily, had better results in terms of adherence to antiretroviral therapy, as it was indicated by patients on stavudine group had better adherence than zidovudine group [37]. This is because patients, who are required to take medicines for their remaining life time, as well, need to be involved in other social and/or economic activities; therefore they need simple regimens that can suit their daily life cycle. Complicated regimens interferes their daily activities with the ultimate drug taking interruptions hence treatment failure [37,38]. Our findings were similar to many studies that reported the benefit of oncea-day rather than twice regimens [37,39]. Many studies show that better adherence to treatment has better immunological outcome and/or clinical outcomes [40-42]. In our findings, the median adherence was 94% (IQR 94%, 98%), that explains why there were significant increase of CD4+ count over time from baseline to follow up time in both groups. Our findings indicated also that patients with CD4+ cell count less than 350 cells/mm3 had better adherence to antiretroviral therapy. This might be due to the facts that, patients with CD4+ cell count less than 350 cells/ mm3 are more likely to fall sick and tired of illnesses [42]. Their motive is based on getting rid of pain associated with their illnesses; therefore they are more likely to adhere to treatment easily. On the other hand patients with CD4+ cell count above 350 cells/mm3 are less likely to have opportunistic infections; therefore they are more likely to assume they are healed and tend to forget taking medicines on regular basis [38]. Our findings were similar to findings reported by Ledergerber et al., Robbins et al. and Muya et al. [40,43,44] who indicated that patients with low CD4+ cell counts are more likely to adhere to treatment regimens . Our study did not show any significant differences between sex and age in terms of adherence, but older (>or=40 years) was associated with good adherence than young age. This is simply because older age are more likely to be involved in taking care of their families, therefore they aspire to be alive and healthy so that can continue working and obtain income for family as well as raising children to their expectations [41,45].
In conclusion, stavudine 30 mg once-a-day has similar immunological and clinical outcomes compared to standard zidovudine dose. Stavudine based regimens have the roles to play in the resource constrained countries. Adherence to treatment was better in a once-a-day dosing group compared to the twice-a-day group.
The limitation of our study was short duration of follow up, this might have not explored enough the long term stavudine (30 mg in a day) related side effects at the proposed low dose. It is our opinion that a longer follow up period should be recommended in order to gather information in relation to long term effect of recommended regimen.
The investigators are grateful to study participants who were bold enough to have a high clinic attendance for the study period of six months. We also acknowledge the health staff team who participated in data collection. The study was funded by Fogarty International Centre (FIC) of the National Institute of Health (NIH) through International Clinical Operations Health Research in Tanzania (ICOHRTA) a collaborative project between MUHAS (Tanzania) and the Harvard School of Public Health (USA).
There were no potential conflicts of interest disclosed.
Download Provisional PDF Here
Article Type: Research Article
Citation: Buma D, Bakari M, Fawzi W, Mugusi F (2015) Clinical and Immunological effects of a Reduced Daily Dose of Stavudine among Antiretroviral Naïve HIV-infected Individuals in Dar es Salaam, Tanzania: A Randomized, Controlled Study. J HIV AIDS 1 (1): doi http://dx.doi.org/10.16966/2380-5536.103
Copyright: © 2015 Buma D et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access