
Figure 1: Shows the effect of arsenic infection on human health

Ashish Kumar11 Mini Namdeo1* Rama Mehta2 Vijaya Agrawala1
1Centre of Excellence-Nanotechnology, Indian Institute of Technology, Roorkee-247667, Uttarakhand, India*Corresponding author: Mini Namdeo, Centre of Excellence-Nanotechnology, Indian Institute of Technology, Roorkee-247667, Uttarakhand, India, Tel: +917060214491; E-mail: mini.namdeo@ gmail.com
Thousands and thousands of people are suffering from the toxic effect of arsenic in many countries. It is due to natural ground water contamination as well as industrial hazards waste and drainage problems. Permissible level of arsenic in water is 0.01 ppb defined by World Health Organization. The delta region of Brahmaputra and Ganga is one of the world’s most affected areas. The central portion of Argentina is affected by arsenic-contaminated groundwater. Specifically, the La Pampa produces water containing 4-5300 microgram as per litre. There are many public water supply systems in United State of America. Their water supply from groundwater had met the old 50 ppb arsenic standard but exceeded the new 10 ppb. China, Australia, Japan, Nepal and many more countries in all over the world are facing problem of ground water contamination of arsenic. There are lot of effort has been made by WHO, NGOs and governments of different countries. But still several cases are found in all over the world. Bangladesh is a most affected country among all of ground water contamination of arsenic.
To make water portable for human consumption, there were several techniques used to remove contamination. Use of chlorine, ozone etc. and physical method such as UV treatment, other filtration techniques such as reverse osmosis, membrane filtration, flocculation, adsorption etc. are some of the effective methods for removal of contamination from water. In this review, some of the most effective arsenic removal techniques will have been discussed.
Arsenic; Contamination; Filtration; Selectivity; Adsorption
Arsenic that has the symbol ‘As’, and atomic number 33, atomic weight 74.92 is often referred to as a metal but, it is classified chemically as nonmetal or metalloid belonging to Group-15 of the periodic table. The most common oxidation states for arsenic are: –3 (Arsenide: usually alloy like intermetallic compounds), +3 (arsenite (As (III)), and most organ arsenic compounds), and +5 (Arsenates (As (V)): the most stable inorganic arsenic oxy compounds). Arsenic is always present as compounds with oxygen, chlorine, sulphur, carbon and hydrogen on one hand, and with lead, gold and iron on the other [1]. It can exist in inorganic or organic form; inorganic arsenic is more toxic than organic arsenic. Inorganic arsenic occurs naturally in many kinds of rocks and it is most commonly found with sulphide ores as arsenopyrite. Inorganic arsenic compounds are known to be human carcinogenic and organic compounds are typically white to colourless powders. Elemental form of arsenic is insoluble in water. It can only soluble in oxidized form [1].
Arsenic, being a normal component of human body is transported by the blood to different organs in the body mainly in the form of mono methyl arsenic (MMA) after ingestion [2]. It causes variety of adverse health effect to human after acute and chronic exposures such as dermal changes (pigmentation, hyperkeratosis, and ulceration), respiratory, pulmonary, cardiovascular, gastrointestinal, haematological, reproductive, immunologic, Geno toxic, mutagenic, and carcinogenic effects [3].
Many countries all over the world are facing problem of arsenic contamination. There are 19 states in India having arsenic contaminated water including Rajasthan, Bihar, Bengal, Utter Pradesh, Orissa, and Gujarat. Severe problems due to arsenic contamination in water have been seen in Rajasthan, Jharkhand, West Bengal and Chhattisgarh [1].
The worst situation of arsenic contamination has been seen in delta region of Brahmaputra and Ganga rivers. Bangladesh is grappling with the largest mass poisoning of a population in history because groundwater used for drinking has been contaminated with naturally occurring inorganic arsenic. It is estimated that of the 125 million inhabitants of Bangladesh between 35 million to 77 million are at risk of drinking contaminated water [4]. The scale of this environmental disaster is greater than any seen before; it is beyond the accidents at Bhopal, India, in 1984, and Chernobyl, Ukraine, in 1986.The effect of arsenic contaminated water as a drinking water on health of human beings in India has been shown in Figure 1 [5]. The situation of this contamination of arsenic in groundwater is not only found in Asian countries but also in Europe, Africa, North America, and Australia.
In this review paper, all the sources of ground water contamination with arsenic has been observed and marked. Affected regions of arsenic contaminated groundwater have also been seen with their effect on human health. Different techniques for arsenic removal from ground water contamination have also been reviewed with their pros and cons. There is lot of techniques are available for the purification of water since very past. But very few are having better efficiency for arsenic removal from contaminated drinking water. Some of those techniques have been mentioned in this review paper like Reverse Osmosis, Activated Alumina, Ferric Activated Carbon, Coagulation and flocculation, Membrane Filtration, Solar Oxidation Technique and FeO nanoparticles. These techniques are having good result against arsenic contaminated water along with the problem of disposal of their hazards residual. In some of the countries, these hazards residuals with arsenic contamination have been used in buildings of roads and in bricks manufacturing [6].

Figure 1: Shows the effect of arsenic infection on human health
Most of the cases of arsenic toxicity in the medieval and early modern age were due to arsenic intake through medicine, smelting or genocide activities. Around the middle of 20th century arsenic poisoning surfaced from some countries where people ingested arsenic contaminated water [7]. The toxicity manifested on mass scale rather than the mere individual cases. The major affected countries were Argentina, Chile, Mexico, and Taiwan. Close to the end of 20th century groundwater arsenic contamination and sufferings of people came to the lime light from three more Asian countries (West-Bengal- India, China and Bangladesh). The source of arsenic was contaminated hand tube wells. In global arsenic contamination scenario 38 countries are affected at present [8]. In Asia alone 13 countries are arsenic affected and Asian countries are worse arsenic affected in global scenario. In Bangladesh alone out of its 64 districts, 60 districts have groundwater arsenic contamination above WHO guideline value (10 ppb) [7]. In India, flood plain regions belonging to Ganga and Brahmaputra rivers are arsenic affected. There are major three types of contamination of groundwater in all over the world; which are given below in Figure 2. On the other hand, affected groundwater regions were given in figure 3. The coloured spot in Figure 3 [9] has specified as three different types of sources of contamination.
Participation of human activities in the contamination level of arsenic in groundwater is increasing day by day. Now in the present scenario of 21th century, human activities are also playing a major role. The major sources of human activities are use of pesticides for crops, medicines, waste product of factories, drainage and thermal power plants etc. A recent example of contamination in this category has found in Rajghat and Badarpur area of Delhi NCR (India) [10,11]. Arsenic contamination located in parts of Delhi NCR, the capital of India in the vicinity of Rajghat and Badarpur coal based thermal power plant with total generation capacity of 1085 Mega Watt. Arsenic, being a very coalphile element, has strong affinity to coal matter. There are instance of major dust pollution around power stations from fly ash dispersal. The main method of disposal of flies ash from the power stations by mixing with water; the resultant slurry is pumped through pipes to ash disposal ponds [12]. An example of human added contamination or anthropogenic contamination has been shown in Figure 4 [13] and Figure 5 [14]. The waste hazards materials from industries were exposed to environments. These hazards disposals are having long time effect on environment equilibrium [15]. In some countries, waste materials from factories flows to rivers without any proper filtration [8]. These are one of the main causes in increasing of arsenic contamination in rivers water and later on it contaminates groundwater of delta regions of that river.

Figure 2: Types of Arsenic Contamination in Groundwater

Figure 3: Shows the types of groundwater arsenic contamination and their sources

Figure 4: Shows an example of anthropogenic contamination of arsenic

Figure 5: Shows the example of arsenic disposal in air
Threats to water quality are divided among agriculture, spills, leaking underground storage tanks and septic systems, urban runoff, mining, and industrial operations. California leads in agriculture, farming and ranching in United State of America, accounting for $20 billion in revenues per year. So it is not surprising that agriculture has emerged as one of the biggest source of groundwater contamination in California. California uses the most pesticides and fertilizers of all the states of United State of America et al Anthony Saramento, April, 2002 [16].
Geothermal activities in the Core of earth cause imbalance on the surface of Earth. These imbalances cause a lot of natural disasters on Earth’s surface like volcano eruption, Tsunami, weathering of rocks and minerals, tornadoes etc. Some of these disasters activity of nature are sources of arsenic contamination in water like volcano eruption, weathering of rocks and minerals [12].
The transport and distribution of arsenic in the environment is complex. Due to the many chemical forms in which it may be present and because there is continuous cycling of different forms of arsenic through air, soil and water. Arsenic dissolved in water can be present in the different form. In well-oxygenated water and sediments, nearly all arsenic is present in the stable form of arsenate (V). In some cases arsenite (III) and arsenate (V) forms are less stable and are interchangeable, depending on the chemical and biological conditions [16]. Some chemical forms of arsenic adhere strongly to clay and organic matter, which can affect their behaviour in the environment that has shown in Figure 6 [17]. Weather rock and soil containing arsenic, may be transported by wind or water erosion. Arsenic releases in to the atmosphere by volcanic activity and attaches to particles that are dispersed by wind and fall back to the ground [18].
Water percolating through soils picks up naturally occurring minerals, salts and organic compounds. As the water migrates downward, the concentrations of dissolved minerals and salts typically increases, a process known as mineralization. In some cases, the percolating water accumulates minerals concentrations high enough that the groundwater no longer used as a water supply. This is also a way of groundwater contamination of arsenic [19,20].
Arsenic undergoes a series of biological transformation in the aquatic environment, yielding a large number of compounds, especially organoarsenicals [21]. Certain reactions, such as oxidation of As (III) to As (V), may occur both in presence and absence of microorganism, whereas other reactions such as methylation, are not thermodynamically favourable in water and can occur only in presence of organism, which indicates that many aquatic organism are capable of accumulating arsenic and may catalyse the oxidation of As (III) to As (V). Biological transformation is significantly important in marine ecology.
In Figure 7 [22], the scenario of contamination and level of arsenic contamination in all over the world has been shown. The intensity of the contamination is increasing year by year due to high need of water consumption to compete the population growth. Levels of arsenic in rice grain are typically 0.05-0.4 ppb for North America, Europe, and Taiwan [23].

Figure 6: Shows contamination of air and water due to volcano eruption

Figure 7: Shows the arsenic affected regions of World
The digging of tube-wells for drinking water supply into aquifers elevated in arsenic in Bangladesh and West Bengal has been described as the greatest mass poisoning in human history, with 36 million people exposed to elevated arsenic in their drinking water [24]. In Bangladesh, arsenic contamination level in groundwater is up to 2 ppm. Groundwater is used extensively to irrigate rice crops in Bangladesh, particularly during the dry season with 75% of the total cropped area are for rice cultivation and 83% of the total irrigated area used for rice cultivation only. Background levels of arsenic in soils from limited surveys conducted in Bangladesh rice paddy field ranges from 4 to 8 mg of arsenic kg-1 [23,25].
There has been considerable investigation into drinking water contamination in Bangladesh/West Bengal, with increasing numbers of epidemiological studies. However, to date, no studies have been published which consider other potential arsenic exposure routes to these populations [26,27]. Food surveys on the daily arsenic intake in the United States and Europe showed that fish products and rice are major dietary source of arsenic. In countries that have a rice subsistence diet, the importance of dietary exposure to arsenic through rice could be considerable. Arsenic levels in rice grain reached 0.7 ppb in rice grown on paddy soils containing 68 ppb arsenic in China, showing the potential for arsenic contamination of rice grain from contaminated paddy soils [28,29].
In Japan, arsenic levels were found to be high in geothermal waters and springs first time in 1950s, and arsenic contamination of groundwater was noted in 1994. Exposure to arsenic from industrial sources was noted in early 1950s. In Iran, chronic arsenic poisoning was noticed and subsequent well-water sampling revealed concentrations exceeding 1,000 ppb in 1981 AD [30].
In India arsenic contamination first foot print are reported in Panjab, Haryana, and Himachal Pradesh. Chandigarh (India) and its surrounding area were first highlighted in 1976 AD as arsenic contaminated ground water. In 1984 AD ground water arsenic contamination was identify in lower Ganga plain of West Bengal. In 1992 AD arsenic groundwater contamination in lower plain area (Terai) of Nepal came to notice [30]. In 2002 June, arsenic contamination located in Bihar in middle Ganga plain and at the same time apprehended contamination in Utter Pradesh lying in middle and upper Ganga plain. During October 2003 AD to August 2005 AD three districts of Utter Pradesh namely, Ballia, Gazipur and Vranashi are present in hit list [25].
In January 2004 AD, 17 villages of Sahebganj district of Jharkhand state of India, in the middle Ganga plain are reported affected. Simultaneously January-February 2004 AD in Assam state of India two districts had an arsenic concentration above 50 ppb. All the states and countries surveyed in the Ganga-Meghan-Brahmaputra (GMB) plain, which has an area of approximately 500,000 Km2 and a population over 500 million, are at risk from ground water arsenic contamination [31].
Bihar: Initially detected in the year of 2002 AD from Semaria-Ojhapati village of Bhojpur districts. 57 blocks in 15 districts are under high arsenic contamination risk [32,33].
Chhattisgarh: Arsenic contamination in groundwater is reported along North-South trending 80 Km stretch of Kotri lineament from Chhattisgarh State. The severity is found in certain part of Ambagarh Chowk block of Rajnandgaon [32].
Jharkhand:Arsenic in water (>0.05 ppm) are reported from 3 blocks of Sahebganj district of Jharkhand. Most affected regions of Jharkhand are Rajmahal, Udohwa and Sahebganj districts. These districts have reported high level of arsenic contamination in groundwater.
Arsenic contamination in India is mainly geogenic and mostly occurs in unconsolidated sediments except in Chhattisgarh. In Chhattisgarh, it occurs in aquifers in Precambrian rocks [34].
The discoveries of arsenic contamination of groundwater have occurred over a span of nearly 100 years, the most recent within the last decade. Observations of health problems first led to the realization that arsenic was being inadvertently ingested. Arsenic poisoning in humans in Argentina was recognized as early as 1913 AD, and attributed to the drinking of groundwater. A possible connection between skin cancers and drinking water was recognized in Taiwan during the 1930s. In the 1940s, arsenic contamination of well water in the Pannonian Basin in Romania and adjacent Hungary was discovered [35]. Recognition of similar occurrences in other European countries such as south-western England, Germany, Greece, and Spain followed, and groundwater with As concentrations that exceed standards are now observed in more than 70 countries worldwide (Nordstrom, 2002; Ravenscroft et al., 2009) [35].
The European guideline for arsenic in drinking water is in accordance with the WHO guideline of 10 ppb. In their background document, the WHO states that the 10 ppb guideline is based on practical considerations (detection limit and feasibility/cost of arsenic removal) instead of the health effects [36]. Arsenic in drinking water supply has never been a matter of interest in most European countries because the standard of 10 ppb is hardly ever exceeded. Nevertheless, in countries such as Hungary, Serbia, Croatia, Greece, Italy and Spain, elevated arsenic concentrations have been detected and special treatment steps are needed to reduce the arsenic to acceptable levels [37]. Arsenic exposure in Hungary, Romania and Slovakia was extensively studied and elevated arsenic exposure via drinking water was found prevalent in some of the studied countries. The median lifetime concentrations were estimated to be 13.3 ppb in Hungary, 0.7 ppb in Romania and 0.8 ppb in Slovakia. Overall 25% of the population was found to have average concentrations over 10 ppb and 8% with exposure over 50 ppb [38].
During the mid-20th century, instances of Arsenic contamination of groundwater were reported for the western USA and Alaska, but were not fully recognized in the states of Oklahoma, Texas, and Arkansas and in the Midwest and North‐ eastern parts of the country until the 1980s [37]. Most recently, groundwater containing arsenic in excess of USA Federal and State MCLs was found in parts of the Atlantic Coastal Plain. In Canada, arsenic contaminated groundwater in New Brunswick and Nova Scotia were noted in the 1970s, and instances in western Canada were noted in the 1960s and 1980s [39].
West Millard County is a desert area of Utah with a low density of population; around 250 people have been drinking well-water with arsenic concentrations of 180-210 ppb, the predominant arsenic species being As (V) (86%) [39]. Researchers were examined for specific signs of arsenic toxicity, including dermal signs. Typical signs and symptoms of arsenic intoxication were not found in any study participants. Participants from Deseret had the highest average concentration of arsenic in urine at 211 ppb and that of Hinckley participants had 175 ppb compared to controls from the Delta (48 ppb) [40]. The highest average concentration of arsenic in hair was 1,210 µg/kg from the Hinckley residents and that of Deseret residents was 1,090 µg/kg compared to controls from the Delta (320 µg/ kg). It was reported hypertensive heart disease, nephritis, neprosis, and prostate cancer among the people of the arsenic affected areas in Utah. Western Oregon Wells in Eugene, Creswell, and Grove districts in Central Lane County, known to yield arsenic-rich groundwater, are in an area underlain by a particular group of sedimentary and volcanic rocks, which geologists have named the Fisher Formation [41]. Lessen County, California in Lessen County, California, arsenic poisoning by well water containing arsenic in the range of 50-1,400 ppb was reported during 1970 ppb. It was found that, for drinking-water with arsenic exceeding 50 (± 30) ppb, there was an increase of arsenic content in hair, indicating body burden due to arsenic exposure. The literature reveals that the health of these people who were exposed to arsenic was not adversely affected. In New Hampshire, concentrations of arsenic were measured in 992 drinkingwater samples collected from randomly-selected New Hampshire households. Concentrations of arsenic up to 180 ppb were found, with water from domestic wells containing significantly more arsenic than that from municipal sources [42]. Water samples from drilled bedrock wells had the highest concentrations of arsenic, while samples from surface wells had the lowest concentrations. The arsenic in groundwater of New Hampshire was derived from weathering of bedrock materials and not from anthropogenic contamination. Analysis of rock digests indicates concentrations of arsenic upto 60,000 µg/kg in pegmatite with much lower values in surrounding schist and granites rocks. Arsenic concentration was found higher 50 ppb in well-water and springs of Fairbanks, Alaska [43]. A study was initiated to evaluate the arsenic content of streams and groundwater of the Pedro Dome summit area, about 30 km north of Fairbanks, Alaska, in the heart of the historic Fairbanks mining district. Arsenic was associated with gold mineralization and is believed to reach the groundwater through weathering of arsenic containing rocks [44].
Arsenic pollution of glacial and fluvio-glacial aquifers is well-known in the USA, Canada and Finland, but largely unrecognised in other glaciated regions. However, the majority of cases come from stable continental regions. Wells drilled on deposits of the youngest glacial advance in the USA (the Wisconsin) were much more likely to be polluted in Minnesota, Iowa and the Dakotas [45]. In Finland, arsenic polluted groundwater was correlated with arsenic anomalies in glacial sediment. However, in most countries of interest here, the requisite geochemical atlases probably do not exist, and it would be simpler, and far more reliable, to survey groundwater directly [46].
Australia is a country rich in minerals that constitute a significant source of arsenic contamination to the environment, in addition to anthropogenic sources, such as mining activities and pesticide usage [47]. In 1991, survey data revealed elevated levels of arsenic in surface water and groundwater of Victoria, mostly around the gold-mining regions. In that area, concentrations of arsenic in groundwater varied from 1 to 300,000 ppb while in surface water these were in range of 1 to 28,300 ppb. In a follow-up study in the same area in the mid-1990s, concentrations of arsenic were 1-12 ppb in groundwater samples, 1-220 ppb in surface water samples, and 1-73 ppb in drinking-water samples [47].
In Australia, old stocks of lead arsenate used as pesticides before 1970 AD remained in sheds and caused chronic arsenic poisoning among workers. Recently, Smith et al. summarized the environmental behaviour of arsenic, with particular emphasis on sources, distribution, and accumulation of arsenic in the Australian environment. They reported the presence of both anthropogenic and naturally-occurring arsenic [48].
In Brazil, concerns were raised about reports of human exposure to arsenic in drinking-water as a result of gold-mining in the zone of MinasGerais in south-eastern Brazil [49]. In 1998 AD, urinary arsenic was measured in 126 school children, and a mean concentration of 25.7 (range 2.2-106) ppb was noted. Environmental investigations in the surrounding regions found that the mean level of arsenic in surface water was 30.5 (range 0.4-350) ppb; levels of arsenic in soil ranged from 200 to 860 mg/ kg, and sediments had a mean concentration of 350 (range 22-3,200) mg/ kg [50].
Several techniques are available in the present scenario of 21st century. All the available techniques are having their own pros and cons as well. Some of them found more practical to use frequently among the mass of arsenic contaminated people. Microbial contamination from water consumption, there are several techniques are used to remove contamination like use of chlorine, ozone etc., physical method such as UV treatment, other filtration techniques such as reverse osmosis, membrane filtration, flocculation, adsorption etc. Some efficient and practical techniques for the purpose of arsenic removal from drinking water will have discussed in this section [6].
This work addresses pressure driven membrane separation processes. Pressure driven processes can be divided into four overlapping categories of increasing selectivity: microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and hyper-filtration or reverse osmosis (RO). It should be noted that, in general, driving pressure increases as selectivity increases [51]. Clearly it is desirable to achieve the required degree of separation (rejection) at the maximum specific flux (membrane flux/driving pressure). In general, separation is accomplished by MF membranes and UF membranes via mechanical sieving, while capillary flow or solution diffusion is responsible for separation in NF membranes and RO membranes. However membrane composition combined with solvent and solute characteristics can influence rejection via electrostatic double layer interactions or other hindrances [52].
When a solution containing ions is brought in contact with membranes possessing a fixed surface charge, the passage of ions possessing the same charge as the membrane (co-ion) can be inhibited. This condition is termed Donnen Exclusion. More specifically, when a solution with anionic arsenate is brought in contact with a membrane possessing a fixed negative charge, the rejection of arsenate may be greater than if the membrane was uncharged [53]. Hence, the selection of a membrane possessing a slight negative charge may be advantageous for the removal of arsenic from drinking water. This is a particularly fortunate set of circumstances, since most NF and UF membranes possess a slight negative charge, and the speciation of arsenic in natural waters is primarily in the anionic arsenate form [51]. If the barrier (membrane) is intact, no particles larger than the membranes pore size can pass through the filter. This is illustrated in Figure 8 [54].
In this method, the activated carbon (AC) (AC12 × 40, China Calgon) was used in this study. This kind of AC has moisture content of 1.2%, ash content of 10.3%, iodine values of AC adsorption of 1029 mg/g, the hardness of 96.2%, and the density of 480 g/L. Grain sizes of AC were: less than 1.7 mm in diameter (less than 12 US mesh sieve; less than 1.8% by mass) and more than 0.425 mm in diameter (more than 40 US mesh sieve; less than 1.9% by mass) [55]. The virgin activated carbon was firstly rinsed with distilled water to remove dirties, and then was washed by 0.001 M HCl solution to remove all salts precipitated in its pores. Then, the AC was repeatedly washed with the distilled water to remove all traces of the acid. Subsequently, the washed AC was oven-dried at 85° C for 24 h to volatilize the organic impurities, and then was modified by 1 N HNO3 for 5 h at the room temperature [56,57].
FeO/AC adsorbent was prepared by mixing FeCl3 /FeSO4 (molar ratio 2:1) and 5 mol NaOH and remaining for 10 min at the temperature of 70°C and pH value of 9.5, along with the gentle stirring (60 rpm), and then was impregnated into the modified AC [58,59]. The obtained materials were dried in an oven at 100°C for 3 h. The samples were analysed by X-ray diffraction (XRD) (Ni filtered Cu K, λ=1.5418 nm), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and nitrogen adsorption measurements [60,61].
Another approach to produce a more efficient system for the reduction of Cr (VI) was to create highly dispersed Fe phases on the surface of activated carbon. These Fe/AC composites show some innovative aspects:-
The most cost-effective method for removing arsenic from a private water supply appears to be reverse osmosis, commonly called RO. RO can be thought of as filtration at a molecular level. It works by forcing water through a special, selective membrane [64].
Reverse osmosis remove total dissolved solids by forcing the water, under pressure, through a synthetic membrane. The membrane contains microscopic pores which will allow only molecules smaller than 0.0001 micron to pass through. Since the molecules of dissolved metals and salts are large compared to the water molecules, the water will squeeze through the membrane leaving the metals and salts behind [65]. A professional Reverse Osmosis system is capable of removing 90-99% of the dissolved mineral salts from water. The mechanism of reverse osmosis has been well described in Figure 10 [66].
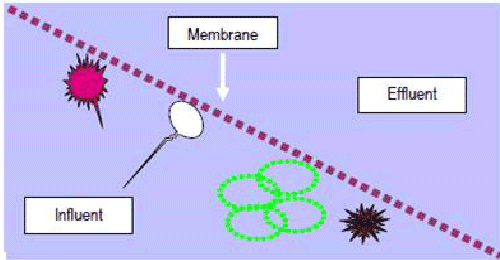
Figure 8: Represents the Membrane Filtration Mechanism
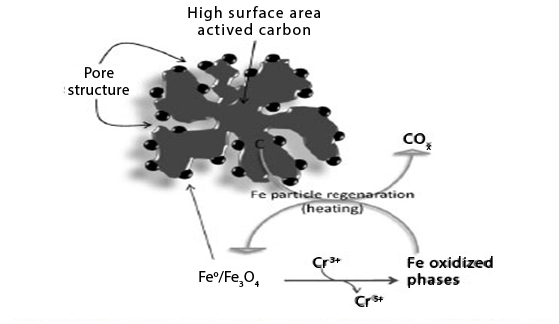
Figure 9: Shows schematic representation of the Fe phases on the surface of activated carbon and process of Cr reduction and Fe oxidized phase regenerations.
Magnetite reduced graphene oxide: Magnetite-reduced graphene oxide (M-RGO) composites (via a chemical reaction with magnetite particle size average of ~10 nm) show very good response against arsenic removal from contaminated water. M-RGO composites are superparamagnetic at room temperature and can be separated by an external magnetic field [67]. These composites show high binding capacity for As (III) and As (V), due to increased adsorption sites in the presence of reduced graphene oxide. The composites show near complete (over 99.9%) arsenic removal within 1 ppb. Thus, they are practically usable for arsenic separation from water [68]. Figure 11 [69] shows different types of interactions in the adsorption of pollutants on metal oxide/graphene oxide [69].
Iron oxide coated sand and limestone: A method for removal of iron and arsenic (III) from contaminated water using iron oxide-coated sand and limestone has been developed for drinking water. Sand was coated with ferric chloride and used as filtering media. Limestone was added onto the coated sand and the effect of limestone addition on removal efficiency of iron and arsenic has enhanced drastically. Maximum removal was found 97.5% with a coated sand dosage of 5 gram/100 ml with limestone by Reshmi and Iohborlang et al December, 2012, [70].
Ferrihydrite, granular ferric hydroxide, and hydrous ferric oxide are the most widely explored iron oxides and hydroxides for the removal of arsenic yielding promising results for both As (III) and As (V) removals [71-76]. In the last decade, removal of arsenic using zero valent iron (ZVI) or Fe (0) for removal of as has been widely explored by several research groups both in the laboratory [77-79] and in the field [80-84]. The removal mechanisms for arsenic and other contaminants using ZVI have been reviewed by Noubactep in great detail [85, 86].

Figure 10: Represents the mechanism of reverse osmosis

Figure 11: Shows different types of interactions is involved in the adsorption of pollutants on metal oxide/graphene oxide
The mechanism of electrocoagulation has been the subject of continual review. The coagulation is brought about primarily by the reduction of the net surface charge to a point where the colloidal particle (previously stabilized by the electrostatic repulsion) can approach closely enough for Van der Waals forces to hold them together and allow aggregation [87]. The reduction of the surface charge is a consequence of the decrease of the repulsive potential of the electrical double layer by the presence of an electrolyte having opposite electric charge. In this process, the coagulant is generated in situ by electrolytic oxidation of an opposite anode material [88]. Charge ionic species arsenic, metals or other components are removed from wastewater by allowing it to react with an ion having an opposite charge or with floc of metallic hydroxides generated within the effluent [89].
The electro coagulation process operates on the principle that the cations produced electrolytically from iron and/or aluminium anodes enhance the coagulation of contaminants from an aqueous medium [90]. Electrophoretic motion tends to concentrate negatively charged particles in the region of the anode and positively charged ions in the region of the cathode. The consumable, or sacrificial, metal anodes are used to continuously produce polyvalent metal cations in the vicinity of the anode. These cations neutralize the negative charge of the particles carried toward the anodes by electrophoretic motion, thereby facilitating coagulation [91]. In the flowing EC techniques, the production of polyvalent cations from the oxidation of the sacrificial anodes (Fe and Al) and the electrolysis gases (H2 and O2 ) works in combination to flocculate the coagulant materials [92]. Even inert electrodes, such as titanium and the passage of an alternating current have also been observed to remove metal ions from solutions and to initiate the coagulation of suspended solids. As mentioned above, gas bubbles produced by the electrolysis carry the pollutant to the top of the solution where it is concentrated collected and removed [93]. The removal mechanisms in EC may involve oxidation, reduction, decomposition, deposition, coagulation, absorption, adsorption, precipitation and flotation [94]. Figure 12 shows electro coagulation process [95].
Solar oxidation in individual units (SORAS) was explored by Garcia et al. [19] as alternative technology to treat arsenic from the groundwater. The process is based on photochemical oxidation of As (III) followed by precipitation or filtration of As (V) adsorbed on Fe (III) oxides. Their findings show that the underlying chemistry is very complex, and the removal efficiency is affected by the changes in the chemical matrix, or by changes in the operative conditions. Eawag, Swiss Federal Institute of Aquatic Science and Technology has currently developed SORAS in its laboratory and field tested in the WATSAN Partnership Project in Bangladesh [96]. However, more studies are requested before this technology is feasible in practical uses of arsenic removal [95]. SORAS reduce arsenic contents to approximately 50 to 80%. The method is conceived for usage at household level to treat small quantities of drinking water at virtually no cost. The working principle of SORAS has been shown in Figure 13 [96].
A liquid and/or solid residual may be produced from an AA system depending on the type of operation. If the system is regenerated, a liquid waste is produced from the backwash, caustic regeneration, neutralization, and rinse steps. In some instances, sludge may be generated from the regeneration and neutralization streams because some alumina dissolves during the regeneration step and may be precipitated as aluminium hydroxide [97]. If aluminium based sludge is produced because of lowering the pH of the liquid residual, this sludge will contain a high amount of arsenic because of its arsenic adsorption characteristics. This sludge and the remaining liquid fraction of the solution will require disposal [98]. Because both residuals contain arsenic, their disposal may be subject to disposal requirements. When the AA has reached the end of its useful life, the media itself will also become a solid residual that must be disposed [99]. Because of its high arsenic removal capacity, an activated alumina system may be operated on a media throwaway basis rather than a media regeneration basis. When operated on a throw-away basis, the exhausted AA media will be the principal residual produced. This media has the potential of being classified as a hazardous waste because of its high arsenic content. A TCLP (Toxicity Characteristic Leaching Procedure) test is necessary, therefore, to determine its classification and ultimate disposal restrictions [100]. Because the AA media will filter out particulate material in the source water, the media bed will occasionally require backwashing. This backwash water will likely contain some arsenic attached to either the particulate material or the very fine AA material that is removed during backwashing. Consequently, the disposal of the backwash water may also be subject to the disposal requirements [101,102].
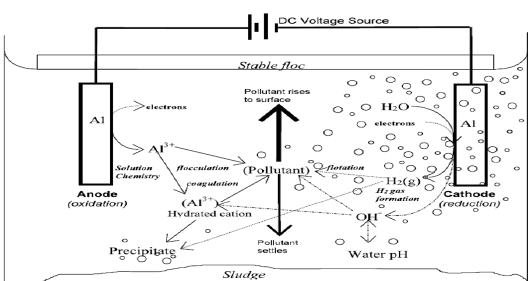
Figure 12: Shows the process of electro coagulation

Figure 13: Shows the example of working principle of SORAS
Nano filtration is also a kind of membrane filtration works on reverse osmosis. It removes molecules and ions effectively. The removal is achieved by the large difference in solution-diffusion rate of water and solute. RO is mainly used for producing desalinated water. Along with harmful ions, it also retains useful minerals. Nano-filtration (NF) process retains large and multivalent ions. NF is also known as “loose RO membranes” due to their relative high water flux [103].
Recently, some of researchers have worked on some more effective methods of arsenic removal. Zhang and Sun, 2013 [104,105], invented multifunctional micro/nanostructured MnO2 spheres successfully and applied them in the removal process of arsenic species from groundwater. As (III) species can be effectively oxidized by the synthesized MnO2 followed by the adsorption of As (V) species [106]. The synthesized material is repudiated with good adsorption and oxidative capacity required for the removal of arsenic species under controlled conditions.
Cui et al. [107], synthesized highly porous, nanostructure ZrO2 spheres from amorphous ZrO2 nanoparticles with the help of a food-safe additive, agar powder, which yielded a simple, cheaper, and safer process for the synthesis of ZrO2 spheres. These ZrO2 spheres displayed good adsorption capacity on As (III) and As (V) at near neutral pH, without the requirement of pre-oxidation and/or pH adjustment of the arsenic contaminated water.
There are a lot of techniques available and a lot more about to discover for the removal of arsenic from portable water. Every technique has their own pros and cons too. The comparison of suitability, percentage of arsenic removal and cost effective like matters have been discussed for these available techniques of arsenic removal. Table 1 has well described the comparison of some effective available techniques.
The problem of arsenic contamination of groundwater in all over the world; from Japan to USA and from Sri Lanka to Russia, are visible and not ignorable. There different types of sources for the arsenic contamination of groundwater. Anthropogenic sources are getting more effective day by day in modern ways of development all over the world. Some countries like Bangladesh, India, Nepal and North American countries are having severe condition of arsenic contamination. A lot of effort has been made by so many Governments and WHO in number of location of World to improve the condition of contaminated groundwater and its severe effect on human health. But mass infected conditions like Bangladesh are not in control and it is going bad day by day. In this review, we have discussed causes of arsenic infection in human food chain.
The best arsenic treatment technique for a given utility will depend on arsenic concentration and the species in source water, other constituents in the water, existing treatment processes, treatment costs, and handling of residuals. Laying techniques where mentioned and discussed in section 4. Some techniques have their benefits along with their back image. For example, Activated Alumina is having good efficiency against arsenic removal from infected water. But the disposal of its hazards residual is prime concern. Reverse Osmosis is good technique. Many of the manufacturing companies of water purifier are using this. But while talking about the mass infected people, it seems that it is unable to fulfil the requirement.
The drinking water contamination is a question on life supporting elements of nature. In this situation the impact will always visible on mass of the people.
The authors gratefully acknowledge Prof. R. Jayaganthan, Head, Centre of Excellence Nanotechnology, Indian Institute of Technology Roorkee, and Dr. R.D Singh, Director, National Institute of Hydrology, Roorkee (Uttarakhand) India for their support and providing facilities. The authors are also want to thank Water Initiative Technology Scheme funded DST, Delhi, India Project No. DST/TM/WTI/2K13/94(G) for the financial support for this work.

Table 1: Comparison of available arsenic removal techniques of arsenic contaminated water
Download Provisional PDF Here
Aritcle Type: Review Article
Citation: Kumar A, Namdeo M, Mehta R, Agrawala V (2015) Effect of Arsenic Contamination in Potable Water and Its Removal Techniques. Int J Water and Wastewater Treatment 1(2): doi http://dx.doi. org/10.16966/2381-5299.108
Copyright: © 2015 Kumar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access