Introduction
Malaria is a mosquito-borne infectious disease caused by protozoan
parasites of the genus Plasmodium which exacts a significant toll of
morbidity and mortality in humans [1]. Around 3 billion people currently
live in malaria-endemic areas, over the last decade leading to an estimated
250 million clinical cases and one million recorded fatalities annually
[2]. In 2013, the most recent year for which data are available, 584,000
deaths and 198 million clinical illnesses were reported [3]. Susceptibility
to severe manifestations of disease is increased in pregnant women and nonbreastfeeding
children under the age of five, especially in endemic areas [4].
The life cycle of Plasmodium progresses through multiple transitions
in alternating hosts, sexual reproduction occurring in the midgut of an
Anopheles mosquito and asexual replication occurring in the liver
and bloodstream of a vertebrate, including humans. It is the latter
cycle of intraerythrocytic vegetative growth and host cell rupture
that is responsible for the pathogenesis of disease and gives rises to the
characteristic influenza-like, paroxysmal symptoms of uncomplicated
infection [1]. When a mosquito ingests a blood meal from a malariainfected
person, male and female gametocyte stage parasites enter the
midgut where they are enclosed by a newly synthesized peritrophic matrix.
Intracellular male gametocytes exit their erythrocytic environment via
a process of exflagellation that is triggered cumulatively by mosquitoderived
xanthurenic acid, a lowering of body temperature and a
commensurate rise in pH, whereupon fertilization soon follows. Within
24 hours a zygote forms and further development and differentiation
results in banana-shaped, motile ookinetes. From the midgut ookinetes
penetrate the epithelium, reach the basal lamina and differentiate into
oocysts. Multiplication through mitotic divisions over 7-15 days may
result in tens of thousands of sporozoites. These migrate to the salivary
glands and are injected into an individual’s body when the now infectious
mosquito next bites a human. Very rapidly after entering the peripheral
blood sporozoites home to hepatocytes and after vegetative multiplication
many merozoites form. These are released back into the blood, invade
erythrocytes and undergo a cycle of asexual replication that repeats
every 46-48 hours for P. falciparum, the deadliest of the human malaria
species. A minority of merozoites leaves this cycle and undergoes sexual
development into male and female gametocytes that are ready to transfer
to a mosquito when it feeds on that person [1]. At each stage different
antigen-specific immunological responses perform protective (and
possibly immunopathological), sterilizing and altruistic effects (Figure
1) [2,5-7]. Harnessing each form of specific immunity plays a role in the
overall strategy to control malaria, such that potentiation of a protective
response underpins rational vaccine design (Figure 2) [8].
The production of an effective vaccine would provide an ideal
addition to the arsenal of tools to combat malaria. While vaccines have
had a dramatic impact on many infectious diseases, to date this is not
the case for malaria, a disease caused by a eukaryotic protozoan parasite
that is much more complex genetically, structurally and physiologically
compared to both viruses and bacteria. This complexity makes it more
difficult to develop a vaccine, exemplified by stage-specific expression of
antigens, phenotypic antigenic variation of cloned asexual erythrocytic
parasites over time and antigenic polymorphism of geographically diverse
isolates [8,9]. Over several decades from the mid twentieth century,
Plasmodium has thwarted considerable efforts to find an effective solution
to the development of an efficacious, commercially available vaccine.
All in all, four recognizably distinct approaches have been taken to
prepare P. falciparum malaria vaccines: (a) a recombinant protein together
with adjuvant for pre-erythrocytic stages of the parasite’s life cycle (e.g.
RTS,S/AS01); (b) whole sporozoite preparations for pre-erythrocytic
stages (e.g. PfSPZ and PfSPZ-CVac); (c) prime-boost constructs that
include recombinant DNA, viruses or bacteria; (d) recombinant protein
combined with an adjuvant for sexual erythrocytic and mosquito stages [9].
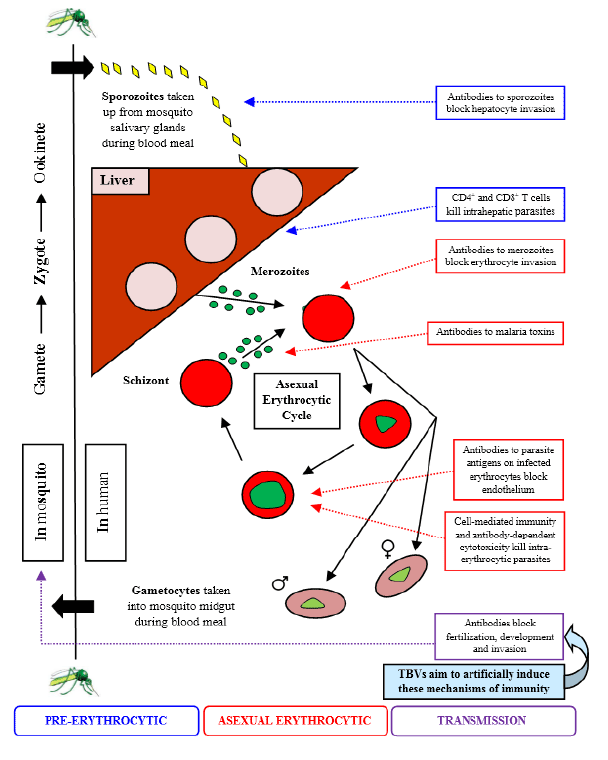
Figure 1: Schematic diagram showing the mechanisms of immunity against different life cycle stages of the major human malaria parasite Plasmodium
falciparum, highlighting those against the sexual stages that transmission-blocking vaccines aims to induce.
Rationale for a transmission-blocking vaccine
Sexual development within the mosquito midgut may represent the
most vulnerable target for vaccines to prevent transmission of malaria
parasites [1]. Such a vaccine would have the potential to reduce the
burden of disease, including in parts of the world’s most malarious
continent, Africa. In Asia and Latin America, it could help lead to the
elimination of the malaria parasite. While the immunization of an
individual with a transmission-blocking vaccine (TBV) cannot directly
prevent that person from becoming infected with malaria via the bite of an
infectious mosquito, if sufficient people in the community are vaccinated
the prevalence of infected vectors will diminish. Hence, this altruistic
strategy would also greatly prolong the useful life of vaccines against other
stages by limiting the spread of parasites that have the potential to become
resistant to these vaccines [10].
In most malaria-endemic locations, a TBV immunization program,
even if partial, would reduce disease and death due to P. falciparum
malaria. In areas of relatively low transmission, as in most endemic
locations outside tropical Africa, disease would be reduced probably in
direct proportion to the effective coverage with TBVs. In many situations
of low endemicity, transmission could be prevented by TBVs. In more
highly endemic regions, the deployment of TBVs in conjunction with
additional traditional measures such as insecticide-impregnated bed
nets could bring the end of malaria transmission within reach. In some
instances even incomplete TBV coverage would slow the build-up of
malaria epidemics and reduce their size very substantially [11].
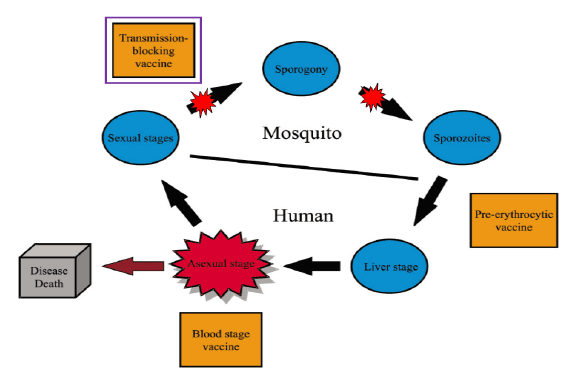
Figure 2: Schematic life cycle of Plasmodium falciparum , showing where vaccination may be expected to intervene. A transmission-blocking vaccine
targets the parasite during its sexual development within the Anopheles mosquito host. In the alternate human host disease pathology is associated
with the cyclical phase of asexual stage replication that occurs in the blood and which causes synchronized erythrocyte rupture and release of pyrogenic
toxins.
Induction of transmission-reducing antibodies was reported first in
an avian malaria model in the late 1950s [12]. Further, in 1976 effective
blocking of transmission was achieved by repeated immunization of
chickens with P. gallinaceum gametes [13,14]. Antibodies were raised
against antigens that were later characterized as P230, P48/45, P28 and
P25 [15], expressed on the surface of gametes, zygotes or ookinetes [16-18].
Several principles guide TBV development [9]. Gamete antigens are an
obvious source of TBV candidates; interference of gamete development
and fertilization provides a source of candidates (such as Pfs25 and Pfs28 in
P. falciparum). Epitopes recognized by transmission-blocking antibodies
may show conservation between geographically distinct parasite isolates
but degrees of variation exist for all candidate antigens. A principal goal of
a vaccine is to induce and sustain a high titer of immunoglobulin G. The
main vaccine targets elicit weak immune responses in primates, so aiming
to boost immunogenicity with adjuvants but without being reactogenic is
important [9].
Targets for transmission-blocking vaccine development
Most current targets for TBVs are either parasite surface antigens,
ookinete-secreted proteins, mosquito components or recombinant
proteins [1]. Each of these categories is discussed below.
Parasite surface antigens: Homologues of the surface antigens first
found in P. gallinaceum were identified subsequently in P. falciparum
and appear crucial to activation of gametes, zygotes and ookinetes. These
proteins have been the primary focus of TBV development, inducing
transmission-blocking monoclonal antibodies against P. falciparum
macrogametes [19,20].
The P. falciparum antigen now known as Pfs230, named for its
molecular weight by SDS-PAGE, and Pfs48/45, another protein named
for its 48 and 45kDa structure on SDS-PAGE [21],are expressed by
gametocytes and appear on the surface of gametes and newly fertilized
zygotes. P230 and P48/45 are known as pre-activation targets [1].
Two other P. falciparum proteins, Pfs25 and Pfs28, are post-activation
targets expressed on the surface of zygotes during their development
to ookinetes [1]. These were identified initially as P. gallinaceum
orthologues using monoclonal antibodies prepared from mice that had
been immunized with ookinetes [16].
Several studies on genetic modification of these crucial proteins have
been performed. For instance, P230 is typified by partially conserved
cysteine motifs paired as cysteine-rich double domains highly constrained
by disulfide bonds [22]. Deleting fragmented or entire motifs from Pfs230
did not affect the role of this protein in gamete emergence although it was
no longer retained on the parasite surface [23].
An interesting feature of the human immune system is the enhancing
effect on transmission-blocking anti-Pfs230 antibodies exhibited by
human complement [24]. However, this complement is degraded by
mosquito proteases within 3-5 hours of blood meal ingestion, so the
window of activity is short [25,26]. It may be that genetic modification
of a mosquito’s protease activity might prove effective in enhancing for a
longer duration the efficacy of anti-P230 antibodies.
Analyzing Pfs230 orthologous genes in eight Plasmodium species
including the human malaria parasite P. vivax showed structural conservation
of the 14 cysteine motif/paired double domains that follow an interspeciesvariable
N-terminal pro-domain [22]. Sequence polymorphisms existed
among the 113 isolates examined. Only a limited number of amino acid
substitutions were found in a subdomain, Pvs230236-943, containing the first
four cysteine motifs. This conserved structure may be the focus of a
candidate P. vivax TBV [22,27]. Again, a comparison of the presence and
absence of human complement was made [27], which showed different
mechanisms of complement fixing by these anti-Pvs230 immune sera [1].
In another study, deletion of Pfs48/45 by gene knockout did not affect
the development of gametes. However, the mutated parasites produced a
significantly lower number of oocysts when fed to mosquitoes [28]. Using
knockouts of the Pfs48/45 orthologue in P. berghei, a rodent malaria
model, confirmed a role for P48/45 protein in fertilization, since male
gametes lacking P48/45 could not fertilize female gametes, whereas they
could still be fertilized by wild type males [28].
By using a single gene deletion of P. berghei Pbs25 or Pbs21, effective
inhibition of ookinete formation was demonstrated, through reduced
parasite penetration into the midgut wall [29]. Of all sexual stage antigens,
Pfs25 and its P. vivax orthologue Pvs25 are the only TBV antigens to be
evaluated in human trials to date. In combination with another seven P.
falciparum antigens Pfs25 was deployed with an attenuated vaccinia virus
vector to generate a multi-stage vaccine candidate named NYVAC-Pf7
[30]; however, immunogenicity was poor and no transmission-blocking
activity by immune sera was detected in the membrane feeding assay that
is used to measure parasite transmission to mosquitoes in the laboratory
setting [9,30].
Ookinete-secreted proteins: In order to continue the life cycle of
Plasmodium within the midgut of the mosquito an ookinete in the
maturation phase that is contained in the blood meal first interacts with
ligands on midgut microvilli, then penetrates into the midgut epithelium
and emerges on the basal side of the cell, coming to rest under the
basal lamina where it forms an oocyst. Each of these processes is totally
dependent upon the activities of proteins secreted from micronemes,
ookinete organelles that have thus become targets for TBV development [1].
The peritrophic matrix in the midgut of an Anopheles mosquito’s
midgut comprises chitins, proteins and proteoglycans. Disruption of gene
expression related to chitinases secretion (Pfcht1 and Pbcht1), paralogous
genes in P. falciparum and P. berghei parasites, respectively, resulted
in remarkable reductions in oocyst formation in mosquitoes [31,32].
Following these revealing studies production of recombinant chitinases as
the basis for a candidate vaccine was instigated.
One of the Plasmodium perforin-like proteins (PPLPs), so-called
membrane-attack ookinete protein (MAOP), which is located in the
micronemes of ookinetes, has been analyzed [33,34]. Disruption of
MAOP gene expression in P. berghei parasites inhibited ookinete
penetration through the midgut epithelium. Further studies on the
activity of antibodies to MAOP should be performed to extend this
evaluation [1]. Moreover, secreted ookinete adhesive protein (SOAP)
and cell-traversal protein for ookinetes and sporozoites (CelTOS) are
two additional ookinete microneme proteins that genetic modification
of the expression of which has a crucial effect on the penetration of
ookinetes into midgut epithelium [35,36]. Subsequent research revealed
that immunization with recombinant PfCelTOS may protect mice against
challenge with lethal P. berghei sporozoites, leading to consideration of
GMP CelTOS as a candidate vaccine antigen [37,38]. Recently, improving
the immunogenicity of PfCelTOS by administration with an adjuvant has
been examined [39].
Mosquito components: Vaccines that target components of the
Anopheles midgut are attractive candidates because the resultant
reduction in vector competence has the potential to simultaneously block
transmission of multiple Plasmodium species [1].
Lectin proteins of mosquitoes, such as jacalin, known to adhere to
glycoproteins of cell walls, reduce ookinete attachment to the mosquito
midgut by binding glycan ligands [40]. A mosquito salivary gland protein,
saglin, was identified as a putative target the blocking of which caused
a 70% decrease in sporozoite levels [41]. By injection of anti-saglin
antibodies into the mosquito haemocoel or disrupting its expression by
RNAi, significant reduction of sporozoites in salivary glands was obtained.
Hence, saglin is considered by some to be a potential target for TBV [1].
Recent analysis suggests that mammalian antibodies delivered in the
blood meal may up regulate the mosquito immune response. Antibodies
to A. gambiae serine protease inhibitor (serpin)-2 reduced P. berghei oocyst
numbers by 54% [42], a finding which warrants further investigation.
Recombinant proteins: On the basis of evidence that P230, P48/45,
P25 and P28 antigens induce antibodies, efforts have been made to attain
the native conformation of these proteins by recombinant technology
in a vector system. In particular, the yeast Saccharomyces cerevisiae has
been used extensively as an expression platform. Initial research showed
that Pfs25 proteins expressed in S. cerevisiae have a partially native
conformation [1]. Trials of a Pfs25 candidate expressed in this system
and formulated with the adjuvant alum were terminated early owing to
reactogenicity, considered likely to be due to an unbound antigen in the
formulation. However, Pvs25 expressed in S. cerevisiae and formulated
with alum was found to be well tolerated in humans [43].
Recently, Pfs25 was expressed successfully in a plant-based vector
system as a fusion to the lichenase carrier molecule (Pfs25eLiKM) [44],
and as a fusion to the Alfalfa mosaic virus coat protein (Pfs25eCP) [45].
In each case, recombinant products were purified to a high level of
homogeneity.
A dual expression system has been developed based on the baculovirus
Autographa californica nucleopolyhedrosis, which possesses strong adjuvant
properties that can activate dendritic cell-mediated innate immunity [1].
This vector offers much promise for future TBV development.
Conclusion
In the continuing drive to produce an effective malaria vaccine, abroad
range of studies has been conducted on antigens expressed during different
stages of the Plasmodium life cycle. Blocking of sexual development
inside the Anopheles mosquito provides a means to combat the parasite
outside of the human body. In this article various potential targets for the
development of TBVs have been described and progress with different
TBV strategies has been reviewed.
Further research is merited to evaluate the added value of TBVs in
reducing or even eliminating locally the spread of Plasmodium, which
would thereby augment the protective effect of anti-malaria vaccines that
target directly parasite life cycle stages in the human host.
Conflict of interest
The authors declare that they have no competing issues of interest.
Acknowledgements
The authors’ research is supported by Central Queensland University and
the Australian Government’s Collaborative Research Networks Program.