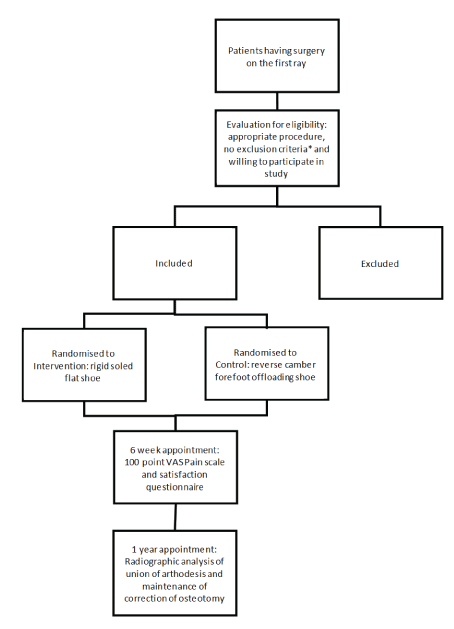
Figure 1: Flow diagram summarising the allocation of participants into groups and the time frame of initial and final assessments.


FORFoot: A Randomised Controlled Trial of Clinical and Radiological Outcomes Comparing Reverse Camber Forefoot Offloading Shoes with Rigid Flat Soled Shoes in Adult Patients with Surgery of the First Ray: Trial Protocol
Forefoot Offloader vs Rigid flat shoe in forefoot surgery (FORfoot)
Robbie I Ray* Paul Dearden Karen Fogarty Andrew P Wines
Sydney Orthopedic Foot and Ankle Research Institute, Sydney, Australia*Corresponding author: Robbie I Ray, Sydney Orthopedic Foot and Ankle Research Institute, Suite G02, Mater Clinic, 3 Gillies Street, Wollstonecraft NSW 2065, Australia, Tel: - (02) 9409 0500; E-mail: Robbie1ray1@gmail.com
Background: Since the advent of modern first ray osteotomies and fixation methods, the need for formal plaster cast immobilization and nonweight bearing status following surgery to the first ray has decreased. Surgeons commonly employ six weeks of postoperative rehabilitation using either a flat or reverse camber postoperative shoe. The flat shoe enables patients to fully protected weight bear with more normal gait whilst the reverse camber shoe allows patient to heel weight bear. There is currently a paucity of clinical evidence to demonstrate if there is a difference between these two postoperative rehabilitation interventions in either patient satisfaction and clinical outcomes.
Methods: This randomised controlled trial proposes to investigate the patient satisfaction with each form of postoperative shoe by analysis of patient reported VAS pain scale and a Likert satisfaction survey. One hundred patients, fifty in each group, will be randomised to receive either flat or reverse camber postoperative shoes. The surgical outcomes will be assessed by radiographic studies at 1 year observing for differences in fusion rates (Hallux rigidus group) and recurrence of deformity (Hallux valgus group).
Conclusions: This study will offer data on the presence of any statistically significant difference in patient satisfaction between the two types of postoperative foot wear and any difference in terms of the clinical outcomes of union rate or recurrence of deformity
Hallux valgus; Hallux rigidus; Postoperative; Shoe; Rehabilitation; Patient satisfaction; Outcomes
Modern stable osteotomies, such as the scarf osteotomy and modern fixation methods for both osteotomies and arthrodesis have diminished the need for plaster cast immobilisation following surgery of the 1st ray [1,2] Special orthopedic shoes which offload the forefoot to varying degrees are commonly used to allow early weight bearing and mobilization [3,4].
One of the most commonly used forefoot offloading shoes is heel weight bearing or reverse camber shoe. This shoe has been shown to be highly effective in reducing forefoot pressures during gait [5,6]. However, wearing an elevated orthopedic shoe causes higher loads in the proximal lower limb joints and increases hip adduction and pelvic tilt on the ipsilateral side, unless there is compensation by an equivalent heel height on the contra lateral foot [7]. There are also concerns regarding instability and falls risk when wearing a shoe with a reverse camber shoe [8].
In patients who have difficulty with a reverse camber shoe due to discomfort and instability we have used a rigid soled flat shoe for postoperative mobilization with good patient compliance and without any obvious detriment to clinical outcome. Whilst this type of shoe has the least effect on gait and is comparable to wearing a normal shoe, [9] there are concerns from pedographic analysis that this type of shoe increases forefoot pressures and may lead to increased postoperative pain and long term problems such as non-union after arthrodesis and loss of correction after osteotomy [4,9].
The hypothesis of this study is that a flat soled shoe is superior to a reverse camber shoe with regards to pain relief and patient satisfaction.
To our knowledge, there are no clinical trials comparing the use of a reverse camber shoe with a rigid flat soled design after surgery of the first ray. Without good clinical evidence, the use of a rigid flat soled shoe cannot be recommended over a reverse camber shoe which is currently the gold standard shoe, used in routine clinical practice [6,9].
This study is designed as a randomised, controlled, researcher blinded superiority trial with two parallel groups, an intervention group consisting of a rigid flat soled shoe and an active control group consisting of a reverse camber shoe. The primary outcome measure will be pain relief at 6 weeks using a 100 point visual analogue scale (VAS) for pain (Figure 1).

Figure 1: Flow diagram summarising the allocation of participants into groups and the time frame of initial and final assessments.
The secondary outcome measures will be patient satisfaction assessed using a Likert scoring system and radiographic assessment at one year comparing rates of union for arthrodesis and maintenance of correction for hallux valgus surgery.
The senior author (AW), a fellowship trained foot and ankle surgeon, will perform all the procedures over two sites. These sites are North Shore Private Hospital and Castlecraig Hospital in Sydney, Australia. The senior author performs around 250 of these procedures each year. However, this included procedures at other sites and bilateral cases which will be excluded from the study. There will be two parallel arms with a 1:1 allocation ratio, containing 50 patients each, comparing a rigid soled flat shoe with a reverse camber shoe as an active control. The study is powered to assess the primary outcome measure, which is VAS pain score at 6 weeks.
Patients will be screened and recruited by the senior author, specialist foot and ankle surgical fellows or the specialist research nurse on admission to hospital/ day of surgery. Eligibility criteria are primary, unilateral surgery of the first ray involving either scarf/akin osteotomy for hallux valgus or arthrodesis for hallux rigidus. Patients will be excluded if they are aged under 18, having bilateral surgery, bunionectomy, cheilectomy, revision surgeryor more complex surgery involving the proximal joints of the foot. Ancillary procedures on the lesser toes are not exclusion criteria. Patients who meet the eligibility criteria will be given a patient information booklet and will have the intervention and control explained to them fully. If the patient agrees to take part in the trial, informed consent will be completed before randomisation
Randomisation will be performed using a computer generated permuted block randomisation system. Allocation will not beformally concealed, however the block randomisation sheet is not consulted until the patient had given informed consent for the study. Given the nature of the study neither the operating surgeon nor the patients can be blinded to the intervention. The early outcomes of VAS pain and satisfaction are patient reported and do not require assessor blinding. When final radiological analysis is performed, the researchers will be blinded to the intervention.
The surgical procedures performed will be a scarf/akin osteotomy for hallux valgus fixed with two 2.7mm screws screws across the scarf osteotomy and a 10mm staple across the akin osteotomy and an arthrodesis for hallux rigidus, prepared with a saw and fixed with two 4mm titanium headless compression and a 3mm titanium neutralisation plate with four 2mm screws. All parameters of the surgical procedure and post-operative instructions will be standardised between groups.
The DarcoMedsurg shoe, which has a rigid sole and an expandable strapless closure that can accommodate postoperative bandages will be used as the trial intervention [10]. The DarcoOrthowedge shoe, which has a reverse camber design and a 15-degree wedge sole to shift body weight to hindfoot and relieve forefoot pressure will be used as the control [11]. Both postoperative shoes are made by the same manufacturer and are similar in design apart from the offloading properties of the Orthowedge shoe.
Patient medical data: All patients will complete a medical questionnaire prior to surgery. Along with simple demographic data such as age and sex this will give the study team data regarding comorbidities which can be used when performing statistical analyses to ensure results are corrected for comorbidities.
Primary Outcomes: A 100 point VAS pain score was chosen as the primary outcome measure as we believe that the current procedures and fixation methods for first ray surgery give adequate stability for healing and that the main purpose of the postoperative shoe is to allow comfortable weight bearing. The patient will be shown how to complete the VAS pain score and encouraged to complete this at the 6 week postoperative appointment.
Secondary Outcomes: A post-operative satisfaction questionnaire has been added as a secondary outcome measure. A bespoke satisfaction questionnaire based on the University of Maryland 100 point painful foot scoring system [12] but formatted to a likertsatisfasction scale and adapted to focus on the postoperative shoe, comfort, stability and ease of mobility will be used Figure 2. The patients will be encouraged to complete this at the 6 week post-operative appointment.

Figure 2: Patient satisfaction as measured using a Likert questionnaire completed at the 6 week appointment following surgery.
A further secondary outcome measure will be union of the arthrodesis and maintenance of hallux valgus correction at one year. Although we feel that our current surgical techniques allow for immediate stability without the requirement for offloading for healing, a major concern of the intervention arm is that a rigid soled shoe will put excessive pressure through the forefoot and affect the overall healing of the first ray procedure. As such after one year, two blinded assessors will review final radiographs and assess union of the arthrodesis in a binomial format and maintenance of correction of hallux valgus angle by comparing this to the radiograph taken at 6 weeks. Patients will be brought back for review at the one year mark and members of the research team who are fully trained specialist foot and ankle fellows will take these radiographic measurements. They will be blinded to the original type of post operative shoe used. Data will be initially gathered on a separate paper questionnaire but will be merged with the originally collected data into a single database for statistical analysis.
Ethical approval and trial registration: This study will be conducted in accordance with the current revision of the Declaration of Helsinki (1996) and the ICH-GCP Guideline (International Conference on Harmonisation, Good Clinical Practice, 1996). The research protocol, patient information sheets and trial consent forms have been reviewed and approved by the North Shore Private Hospital Ethics Committee (NSPHEC 2015-015 for protocol version 1 dated 15 November 2015). The trial has been registered as a controlled trial on the Australia New Zealand trial registry. (ACTRN12617000200381) [13]. This protocol has been produced in accordance with SPIRIT guidelines [14].
All data will be collected in paper format, on a structured data collection form which has been approved by the sponsor and the research ethics committee. The data sheets will be kept in a secure locked filing cabinet and the research assistant will then manually enter the data into a secure password protected spreadsheet disposing of the original paper copies. The data will be kept on an encrypted, sponsor approved USB disk and only accessed by members of the research team. Each patient will have a numerical code so that only when all the data has been transferred to the spreadsheet will the intervention be checked from the initial block randomisation form reducing the risk of bias.
Previous studies have shown that the minimal clinically important difference in a 100 point VAS pain score for musculoskeletal conditions is around 20 points [15,16]. To ensure minor differences in pain were detected we performed a sample size calculation to power the study to detect a treatment effect at 6 weeks between the two types of post operative shoe of 10 points on the VAS pain scale with 80% power at the 5% significance level. A recent study of surgery of the first ray was used to define mean post operative VAS pain and the standard deviation [17]. Our study will require data from 84 patients. We aim to recruit 100 patients to allow for around 15% loss to follow up.
Statistical package for social sciences version 20 (SPSS Inc., Chicago Illinois) will be used for data analysis.VAS pain scores will be assessed for normality using the Shapiro-wilk test. If the data is normally distributed an Independent t-test will be used to analyse results otherwise the MannWhitney U test will be used. The satisfaction score will yield ordinal data and as such the Mann-Whitney U test will be used. For radiographic analysis, a Chi squared test will be used to analyse the dichotomous data regarding union of the arthrodesis and normality testing and appropriate parametric or non parametric tests will be used to assess the maintenance of hallux valgus angle following osteotomy. Furthermore, corrections will be made for age, sex, smoking status and comorbidity with diabetes or peripheral vascular disease as these can affect outcomes after forefoot surgery
The aim of this study is to investigate the impact of two different postoperative rehabilitation regimens on patient satisfaction. Clinically relevant outcomes will also be assessed namely differences in union rates in 1st MTPJ fusion patients and degree of post surgical deformity recurrence in hallux valgus patients.
This trial is currently recruiting participants. The first patient was recruited in February 2016. The final patient is recruitment is expected to be complete by June 2017.
Download Provisional PDF Here
Article Type: Research Article
Citation: Ray RI, Dearden P, Fogarty K, Wines AP. (2017) FORFoot: A Randomised Controlled Trial of Clinical and Radiological Outcomes Comparing Reverse Camber Forefoot Offloading Shoes with Rigid Flat Soled Shoes in Adult Patients with Surgery of the First Ray: Trial Protocol. Forefoot Offloader vs Rigid flat shoe in forefoot surgery (FORfoot). J Orthop Clin Stu Adv Res 1(1): doi http://dx.doi. org/10.16966/2576-6449.101
Copyright: © 2017 Ray RI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access