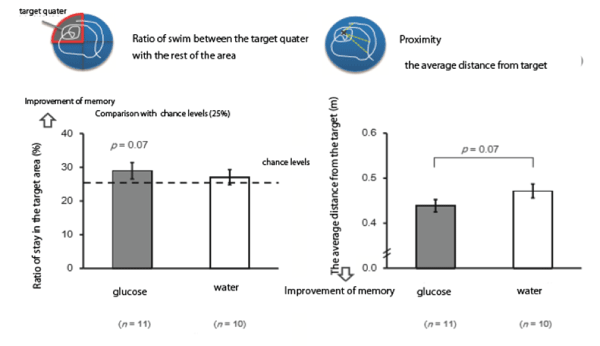
Figure 1: Effects of glucose administration on the improvement of memory-which shows that the ratio of stay in the target quarter to the rest was larger after glucose administration, but not significant.


Akikazu Takada1* Mutsumi Ogawa2
1International Projects on Food and Health (NPO), Tokyo, Japan*Corresponding author: Akikazu Takada, International Projects on Food and Health (NPO), Sumida-ku Ishiwara 1-30-6-802, Tokyo 130- 0011, Japan, Tel: 81-338291849; Fax: 81338291847; E-mail: takadaa@mwd.biglobe.ne.jp
Background: Effects of sucrose administration on brain functions were not elucidated well.
Methods: Spatial memory tests were performed for rats using Morris-maze after administrations of glucose or sucrose. In human, glucose, sucrose or fructose was given to young women and UchidaKraepelin tests were performed to know the working ability.
Results: In rats, the ratio of stay in target quarter was significantly larger in rats given sucrose than glucose. In humans, there was a significant increase in the working ability measured by UchidaKraepelin test after the administration of sucrose. There was a tendency for increase in the working ability after glucose or fructose administration but not statistically significant.
Conclusion: Sucrose administration improved the memory in rats and the working ability of young women possibly not only by transport of glucose into the brain but the increased activity of the brain hedonic sites by the stimulation of sweet receptors, T1R 23.
I review the works and try to give a hypothesis for the improvement of brain functions by sucrose.
Glucose; Sucrose; Fructose; Glycemic index (GI) Morrismaze; Uchida-Kraepelin test; Memory; Sweet receptor; T1R 23
The energy requirement of the brain is made possible almost exclusively by glucose degradation [1].
The energy requirement of the brain is 20-30% of the whole organism at rest, but its weight is only 2%. Only 30% of glucose is required for the metabolism of the amino acids, lipids and nucleic acids [2].
It has been shown from animal studies that increased blood glucose levels are associated with improved memory and attention. Previous studies have found human memory to be facilitated by the administration of glucose [3-6].
There was a significant correlation between blood glucose values and the number of words recalled. Those whose blood glucose levels were increasing remembered significantly more words than those whose blood glucose levels were falling [7].
Extensive evidence indicates that relatively modest increases in circulating glucose concentrations enhances learning and memory processes in rodents and humans [5]. In rats, systemic injections of glucose enhance learning and memory under many conditions.
Sucrose is degraded to glucose in the intestine and glucose is transported to the blood, but few systematic studies have been carried about as to the effects of sucrose on brain function [6,7].
We have recently shown by using Morris maze experiments that sucrose enhanced memory of rats more significantly than glucose although glucose tended to enhance memory [8,9].
We also showed by using Uchida-Kraepelin tests that the administration of sucrose increased the working ability in young women. Glucose administration tended to improve the working ability, but not significant [10].
I now would like to review roles of glucose and sucrose in brain functions introducing our results in rats and humans.
Gold PE (1992) used a spontaneous alternation task [11]. The apparatus is a Y-shaped maze in which rats are allowed to roam freely for 8 min. As rats move through the maze, they visit the least recently visited arm for successive entries. The memory component in this task is that rats remember which arm they have entered most recently to select a different arm. Rats alternate about 70% under control conditions.
Young mice alternate at 70% rates, Aged mice show a deficit with 50% chance levels. If aged mice receive glucose injections before testing, their alternation scores are near 70% values seen in young mice.
Morris used the delayed matching-to-place task which is an unusual variant of the water-maze protocols [12].
We used this technique to examine effects of self-administration of glucose or sucrose on space memory in rats.
Wistar rats aged 11 weeks were trained in water to learn the location of the platform, Rats learned to reach the target in 15 min. after 6-12 trials. Rats were given either glucose (2 g/8 mL/kg) or 8 mL of water intraperitoneally. 24 hours later rats swam in Morris water maze and the ratio of stay in the target quarter to the rest of the area were measured. The distance to the target was also compared between glucose administered rats and control rats.
These results clearly show that sucrose improved memory consolidation compared with sucralose administration using Morris maze experiments (Figures 1 and 2).

Figure 1: Effects of glucose administration on the improvement of memory-which shows that the ratio of stay in the target quarter to the rest was larger after glucose administration, but not significant.
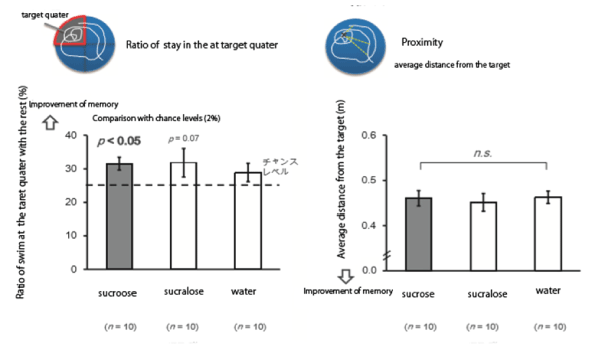
Figure 2: Effects of sucrose administration on the improvement of the memory-shows that rats given sucrose stayed in the target quarter significantly more compared with rats given sucralose. There was no significant decrease about the proximity measurements between sucrose and sucralose given rats.
We asked female college students to participate in the experiments. They were recruited if there were no health problems such as diabetes, hypertension or not serious diseases experienced in the past. They did not smoke in the past. We also excluded people who took drugs for dyslipidemia, hyperglycemia, or hypertension.
We collected blood samples early morning. Participants were asked not to eat anything after 23.00 PM the previous evening. We obtained an informed consent prior to conducting the protocol which had been approved by the Ethical Committee of Showa Women’s University.
Healthy participants were given self-administered diet history food frequency questionnaires based on food groups by recollection of diets they took. From these questionnaires, we calculated the intakes of Energy, Carbohydrate, Fat, and Protein.
Blood glucose levels were measured by using a finger stick (TERUMO kit).
At 9 AM in the morning, blood was taken from fasting participants. They took Uchida-Kraepelin tests and drank 500 mL of solutions containing 50 g. of sucrose, glucose or fructose, or else 500 mL of water. 15 min. after taking drinks, they participated in Uchida-Kraepelin tests, then 30, 60, 120 min. after taking drinks, their bloods were taken.
We took blood at 0 min. and also measured blood glucose levels. Participants are engaged in Uchida-Kraepelin test and they took 500 mL solution containing 50 g of either glucose, fructose or sucrose. As a control they took 500 mL of water.
There are numbers of a digit lined. Two numbers lined together are added. The number of the higher digit is described. This procedure is repeated for 1 min. Then the addition of numbers of the second line was performed and repeated for 15 min. The total numbers added were calculated, and compared before and after the experiment.
The working duty of 1 min. was repeated 15 times then drinks were taken. After blood measurements at 30 min. tests were repeated.
Blood levels of glucose were 138 ± 23 mg/dL for sucrose administration, and 143 ± 25 mg/dL, for glucose administration, respectively. Control level (water) was 82 ± 8 mg/dL. Glycemic index (GI) has been introduced by Jenkins and coworkers in 1981 [14-17] and is defined as the area under the blood glucose curve measured two hours after consuming 50 g of test carbohydrates in relation to 50 g of glucose or white bread.
GI was 88 for sucrose, or 16 for fructose administrations, respectively. Fructose administration did not result in much increase in GI (Figure 3).
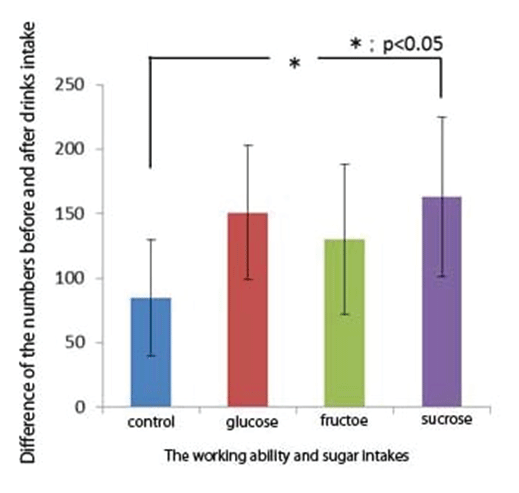
Figure 3: Shows relationship between sugar administrations and the working ability- shows that the working ability was significantly higher after sucrose administration, although there was a tendency for the working ability to increase after glucose or fructose administration, but not significantly.
We examined correlation coefficients between blood glucose levels and the working ability. Although there tends to be increase in the working ability with increase in blood glucose levels, but not significant.
There were many papers which examined effects of glucose injections on brain functions [18-25].
Recently sugar intake is linked to obesity or diabetes. On November 8, 2016, at the Presidential election in US, residents in parts of California’s Bay Area, which include San Francisco, as well as Boulder,Colorado, passed by wide margins a measure to institute a tax on sugar-sweetened beverage [26]. The World Health Organization (WHO) supported such tax in countries across the globe in midOctober, 2016. Such tax was implemented in other US cities as well as in other countries, such as France, Mexico, the UK, the Philippines and South Africa. Proponents of such tax argue that it can lead to marked reductions in the consumption of sugary beverages, and help in the fight against obesity and diabetes.
These arguments take it for granted that there is a smoking gun for relationship between sugar intake and obesity or diabetes.
Recently, there was an article about “sugar conspiracy” in the field of nutrition [27]. An allegation allegation was made against the sugar industry with claims that prominent industry-backed researchers in the 1969s downplayed or suppressed evidence linking sugar and heart disease. After historical investigations, the authors concluded that there was no such thing called “sugar conspiracy”.
We have reported that sugar or sweet beverages intake had no relationship with BMI or blood levels of glucose or insulin [28].
Sucrose is an important factor supplying glucose to the brain. We wondered why sucrose administration is more effective than glucose administration in the improvement of memory or the working ability. Since fructose uptake did not result in increase in blood glucose levels compared with glucose intake, and improvement of the working ability was observed to some extent, we thought taste may contribute to this improvement.
It has been shown that the stimulation of sweet taste resulted in increase in dopamine release in N Accumbens and caused hedonic responses [29]. Fructose receptors of the tongue are T1R 2 3, which is the same for sucrose receptors [30] (Figure 4).
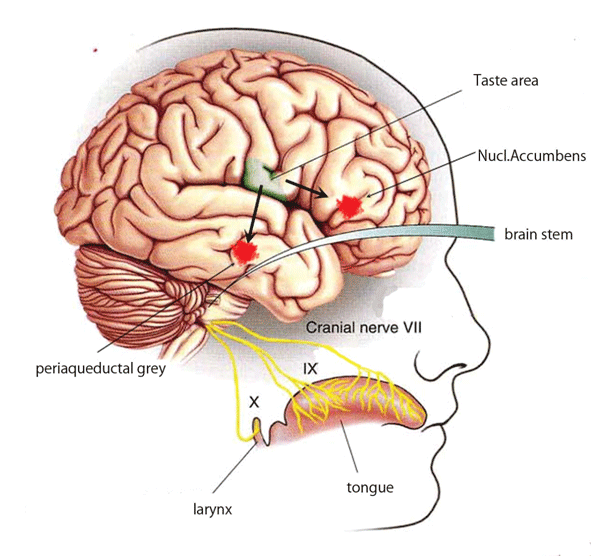
Figure 4: Schematically shows pathways of stimulated taste receptors in the tongue to the brain.
The stimulation of sweet receptors by sucrose may enhance the release of dopamine in the hedonic area together with the release of β endorphin from the periaqueductal grey. These stimulations of the brain areas may contribute to improvement of the working ability and memory more effectively glucose.
There is a report [31] indicating that hyperglycemia (not just T2DM) exerts a specific detrimental influence on cognitive decline in dementia free older adults. In addition, this study indicates that diet based glycemic load is a predictor of poorer cognitive performance.
We agree with this statement. We just say that glucose improves the brain activity in rats and not diabetic humans. Probably, in patients of T2DM increased blood glucose levels may be detrimental because glucose may give damages to neurons which are not seen in healthy neurons. This is the suggestion indicating that hyperglycemia is not good for mental health in patients of T2DM. In the present study, we are discussing the role of glucose in the brains of healthy people.
The results are presented as means ± SD. Statistical significance of the differences between groups was calculated according by one-way ANOVA. When ANOVA indicated a significant difference (p<0.05), the mean values of the treatment were compared using Tukey’s least significant difference test at p<0.05. Spearman’s correlation tests were used to examine statistical significance.
Download Provisional PDF Here
Article Type: MINI REVIEW
Citation: Takada A, Ogawa M (2018) Sucrose and Brain Functions. Obes Open Access 4(1): dx.doi.org/10.16966/2380-5528.135
Copyright: © 2018 Takada A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access