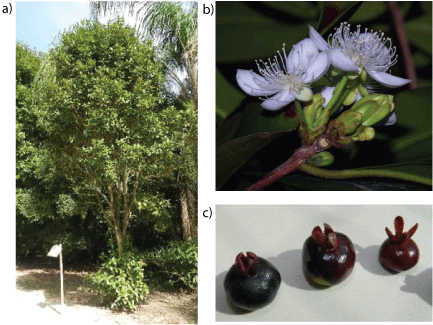
Figure 1 (a,b,c): Grumixama tree in its natural habitat, its flowers and fruits


Nascimento LSM1,2 Santiago MCPA1* Oliveira EMM1 Borguini RG1 Braga ECO3 Martins VC4 Pacheco S1 Souza MC5 Gogoy RLO1
1Embrapa Agroindústria de Alimentos, Guaratiba, Rio de Janeiro, Brazil*Corresponding author: Manuela Cristina P. de A. Santiago, Embrapa Agroindústria de Alimentos, Guaratiba, Rio de Janeiro, Brazil; E-mail: manuela.santiago@embrapa.br
Eugenia brasiliensis Lam., locally known as Grumixama, is a species of Myrtaceae commercially underexploited in Brazil. The study was performed in order to identify and quantify, by high performance liquid chromatography, bioactive compounds such as vitamin C, carotenoids, anthocyanins, flavonoids and phenolic acids in the fruits of this species. The vitamin C content in the edible part was 18.75 mg.100 g-1, classifying this fruit as rich in this bioactive compound. Eight carotenoids were identified and quantified, the main one was β-cryptoxanthin with a content of 22.3 µg.g-1, sufficiently high to consider this fruit as a new source of this carotenoid. Also, 4837.21 mg.100 g-1 of the anthocyanins were obtained in the freeze-dried fruit peels. Furthermore, ten, nine and seven phenolic compounds were identified in the peel, pulp and seeds of grumixama, respectively. The results showed that the fruit of grumixama is promising for exploitation and has the potential for innovative healthy products.
Anthocyanins; Myrtaceae; β-Cryptoxanthin; Phenolic Compounds
Myrtaceae is one of the most important families of Angiospermae in Brazil. However, despite its importance, few species of the family Myrtaceae are exploited; one such unexploited species is Eugenia brasiliensis Lam., popularly known as grumixama.
Native to the Atlantic Forest, this plant is a medium-sized tree, highly resistant to climate change and occurs from the south of the state of Bahia in the north to Santa Catarina state in the south of Brazil. Its flowers are white, quite fragrant, and they are endowed with dense and narrow crowns. This specie blooms from the end of September to November and its fruits usually ripen through the months of November and December. It produces large amounts of fruit every year, but there is no data concerning their productivity, due to the lack of commercial production.
The fruits of E. brasiliensis Lam. are edible and there are three varieties, according to their color: red, purple (or black) and yellow or white [1]. Its thick pulp has a light color, is very sweet and juicy, and normally melts in the mouth, with a flavor similar to that of cherries, which is why they are also called Brazilian cherry. Few studies have been carried out concerning its fruit and the identification of its bioactive compounds. Nine anthocyanins were already identified in grumixama fruit [2]. In fruit of E. brasiliensis Infante et al. [3] studied its phenolic composition as well as its antioxidant and anti-inflammatory activities, not describing other bioactive substances evaluation. Recently Siebert et al. [4] also evaluated its phenolic profile and biological activity, but only from leaf extracts. Such studies are important due to the possibility of finding new sources of biologically active compounds and fostering interest in its commercialization besides creating new healthy products associated with the preservation of biodiversity.
Grumixama is already locally consumed. There is a little commercial production of this fruit, which has been used by high-class restaurants. It is appreciate as a berry fruit, due to its format, color and flavor.
This work aims to contribute to the knowledge of the functional value of the little explored Myrtaceae specie, E. brasiliensis, Lam., known as grumixama. This study is specifically of the black variety, and aims to identify and quantify compounds with antioxidant potential such as carotenoids, flavonoids, phenolic acids, vitamin C and anthocyanins in the whole fruit.
Grumixama fruit (4 kg) was collected in December (summer season) 2014 in the Barra da Tijuca district of Rio de Janeiro, Brazil (23°00’18.8” S 43°25’20.9” W) (Figures 1a-1c). The botanical identification was performed by D.Sc. Marcelo da Costa Souza, professor and researcher at the Institute of Botany of Rural Federal University of Rio de Janeiro. A voucher specimen of the species has been deposited at the Herbarium of Rural Federal University of Rio de Janeiro under number RBR 16004.

Figure 1 (a,b,c): Grumixama tree in its natural habitat, its flowers and fruits
After collection, the ripe fruit was immediately selected and separated into 3 samples with a mean of 300 fruits per sample. After washing, the peel, pulp and seeds of the grumixama fruits were separated, crushed and weighed in triplicate (1 g each) for water content analysis, carotenoids, sugars, ascorbic acid (vitamin C), flavonoids, and phenolic acids. The anthocyanin analysis was performed using the freeze-dried peel (freezedryer L101, Liotop, Sao Paulo, Brazil).
Acetonitrile, acetone, ethyl acetate, methanol, methyl tert-butyl, and petroleum ether were purchased from TediaTM (Ohio, USA), while formic acid (purity=98-100%) was acquired from Merck (Darmstradt, Germany). All solvents were HPLC grade. Phosphoric acid (purity=85%) was also acquired from TediaTM (Ohio, USA). Standards of glucose, fructose and sucrose (purity>95%), ascorbic acid (purity=99%), flavonoids and phenolic acid (purity>95%) were purchased from Sigma Aldrich® (EUA). Standards of carotenoids and anthocyanins were isolated from natural sources at Embrapa Food Technology HPLC Laboratory (Rio de Janeiro, Brazil) with purity>95%. Ultrapure water (0.054 µS cm-1) was obtained from a Milli-Q system from Millipore (Milford, MA, USA). Mass spectrometry grade solvents were used for high performance liquid chromatography with mass detection (LiChrosolv Merck).
Grumixama fruits were individually weighed in order to establish the mean weight per fruit. The fruit parts (seed, skin and pulp) were separated, and each part was individually weighed to define the content percentages (%). This procedure was repeated with six fruits.
Ten fruits per sample were separated in three glass vials, identified as samples A, B and C. The edible fraction (peel+pulp) of fruits was homogenized for the Soluble Solids (SST) and Total Acidity Tests (ATT). The relationship also known as “RATIO” (division of brix degree the amount of titratable acidity) was used to verify that the feedstock is in ripeness such that, resulting in a product with organoleptic acceptance and quality [5].
The soluble solids content was carried out by direct reading in a Digital refractometer (Atago PAL-1 model) of three samples (one reading per sample), with scale in °Brix [6].
For total acidity analysis, 5 grams of each sample were weighed and placed in a 100 mL beaker, and then 20 mL of ultrapure water was added. After checking the initial pH the samples were stirred and titrated in an automatic titrator (Metrohm model 785 DMP Titrino) with 0.05 M Sodium Hydroxide pH 8.1 [6].
Moisture analysis of the fruit parts was performed gravimetrically in an oven at 105°C [7]. The results were expressed in percentage (%).
The carotenoid extraction was carried out according to RodriguezAmaya [8]. The solvent extraction and subsequent saponification of the ether extract were performed with potassium hydroxide (KOH) solution 10% in methanol (10:90, v/v) at room temperature and a reaction time of 16 h in the dark.
The previously weighed sample was transferred to a porcelain mortar, and was macerated with 3 g of celite and 20 mL of acetone. The mixture was vacuum filtered through a glass funnel with a sintered plate. The extraction procedure was repeated until the sample no longer exhibited the characteristic color of carotenoids. The acetone extract was transferred quantitatively to a separator funnel containing 30 mL of petroleum ether and washed, at least five times, with 200 mL ultrapure water. The ether extract was transferred to an amber flask with an equal volume of potassium hydroxide (KOH) 10% methanol (10:90, v /v). After 16 hours, the volume was transferred to a separator funnel and washed with ultrapure water. The ether solution remaining in the separator funnel was transferred into a 25 mL volumetric flask with the aid of a funnel with anhydrous sodium sulfate and glass wool. Finally, the level of total carotenoids in the sample extract was determined by spectrophotometry at 450 nm using an UV-V is spectrophotometer, UV-1800 model 92 (Shimadzu Corporation, Kyoto, Japan), and petroleum ether as blank. The Beer-Lambert law was used to calculate the total carotenoids content.
The chromatographic analysis was performed according to Pacheco et al. [9] using a WatersTM HPLC system, with a photodiode array detector WatersTM model 996, EmpowerTM software, C30 column (S-3 Carotenoid, 4.6 mm × 250 mm, YMCTM) , column temperature of 33°C, and mobile phase in gradient elution mode: methanol (A) and methyl tert-butyl ether (B) starting with 80% A and 20% B; 0.5 minutes 75% A and 25% B; 15 minutes 15% A and 85% B; 15.50 minutes 10% A and 90% B; 16.55 minutes until the final 80% A and 20% with flow 0.8 mL.min-1, injection volume was 15 µL, and the run time was 28 minutes.
Identification of the carotenoids was based on their retention times and absorption spectra UV-Vismax, compared to the retention times of the carotenoid standards based on calibration curves made with analytical standards isolated in the laboratory, with purities greater than 99% [9].
The provitamin A was calculated according to the conversion factor, where 6 µg of β-carotene is equivalent to 1 µg of Retinol Equivalents (RE), with the activities listed as follows: 100% for β-carotene 50% β-cryptoxanthin and α-carotene [10].
The method used for the ascorbic acid analysis was according to Rosa et al. (2007) [11]. The sample was extracted with 10 mL of 0.05 M H2SO4 in an ultrasonic bath for 10 minutes. After extraction, the sample was filtered and an aliquot was transferred to a 1.5 mL vial. Chromatographic analysis were carried out in a Waters® Alliance 2695 system equipped with a WatersTM 2996 Photo Diode Array (PDA) detector (243.9 nm) and a AminexTM BioRad HPX-87H column (300 × 7.8 mm). The autosampler temperature was maintained at 10°C, and the mobile phase of 0.05 M sulfuric acid was pumped isocratically at a flow-rate of 0.7 mL.min-1. External standardization was applied for quantification.
The extraction of sugars was carried out according to Macrae [12]. The sugars were extracted using 1 g of sample and 10 mL of ultrapure water in an ultrasonic bath for 20 min and then the extract was filtered.
This was analyzed by a Waters high performance liquid chromatograph model AllianceTM 2690/5, a Waters refractive index detector model 2410, EmpowerTM software, and a ZorbaxTM Carbohydrate Agilent column (30 cm × 4.6 mm). The flow rate used was 1.0 mL.min-1, the injection volume was 20 µL, and the isocratic elution mode had a mobile phase of acetonitrile: water (75:25 v/v) and a run time of 20 min. Analyses were performed in triplicates.
The quantification of the sugars was performed by external standardization, based on calibration curves made with commercial analytical standards of fructose, glucose and sucrose.
Only the freeze dried fruit peels were used for anthocyanin analysis. Anthocyanins were extracted using 0.02 g of the obtained powder and 10 mL of methanol: formic acid solution (90:10 v/v) in an ultrasonic bath with subsequent centrifugation until discoloration of the sample [13]. Then, an aliquot of the extract was dried and diluted with 100 µL of 5% formic acid solution in water: methanol (90:10 v/v). Analyses were carried out on a WatersTM Alliance 2695 system, using a WatersTM 2996 photodiode array detector (scanning at 210-600 nm with quantification at 520 nm), with a ThermoTM Scientific C18 BDS (100 mm × 4.6 mm; 2.4 µm) column, a flow rate of 1.0 mL min-1, column temperature of 40°C, and injection volume of 20 µL. The elution mode gradient used 5% formic acid (phase A) and acetonitrile (phase B), starting with 95% of phase A and 5% of phase B; 15 minutes 87% A and 13% B; 16.5 minutes 86% A and 14% B; 18 minutes 95% A and 5% B; with a flow rate of 1.0 mL.min-1, an injection volume of 20 µL, and a run time of 20 minutes.
The quantification of the main anthocyanins was performed by external standardization, based on calibration curves made with analytical standards isolated in the laboratory [14]. The main anthocyanins identified were confirmed by mass spectrometer. The other anthocyanins were identified by comparing their retention times and the absorption spectra UV-Vismax with grape peel anthocyanin extracts, which is a matrix already identified for anthocyanins, and by comparison with literature.
The analysis of free phenolic acids was performed according to the method described by Pérez-Jimenéz [15]. The sample was extracted with 4 mL of methanol: water (50:50 v/v, pH 2) followed by mechanical stirring for 1 hour and centrifugation (5000 G) for 10 minutes. The supernatant was collected (extract 1). Then, 4 mL of acetone: water (70:30; v/v) was added to the residue and the mechanical stirring and centrifugation steps were repeated. The supernatant was collected (extract 2). 3 mL of the supernatants (extract 1 and extract 2) were mixed and transferred to a 1.5 mL vial for the chromatographic injection.
The solid samples residues were submitted to the extraction of hydrolyzed phenolic according to the method described by Frighetto et al. [16]. Alkaline hydrolysis was carried on with 5 mL solution of 2 M NaOH containing 1% of ascorbic acid and 10 mM EDTA. This solution was added to the samples followed heating at 61-63° for 60 min. immediately after this 1.5 mL of 6 M HCl was added for the acid hydrolysis. This solution was vortexed for 10 seconds, left to cool to room temperature, and then centrifuged (2700 rpm) for 10 minutes. The supernatant was collected and 6.5 mL of ethyl acetate was added. The organic phase was separated and the extraction with ethyl acetate was repeated. The organic fraction was dried under a nitrogen gas (N2 ) flow and then diluted in methanol for chromatographic analysis.
The analyses of the two fractions (free and hydrolyzed phenolic acids) were performed in a high performance liquid chromatograph Alliance WatersTM model 2690/5, a WatersTM photodiode array detector model 2996 (270, 310 and 370 nm), with a Thermo Hypersil BDS C18 column (100 × 4.6 mm × 2.4 µm), a 1.0 mL.min-1 flow, an injection volume of 10 µL, a run time of 28 min, and the elution mode gradient used an aqueous solution of 0.15% phosphoric acid (95%) and acetonitrile (5%). At 12.00 min the acetonitrile concentration was increased to 12%, at 18.00 min to 20%, and at 20.00 min to 50% acetonitrile. The acetonitrile concentration was maintained at 5% through to 25.00 min and then returned to the initial condition (5%). The quantification of flavonoids and phenolic acids was performed by external standardization.
The mean weight per fruit was 4.5 ± 0.5 g. The grumixama fruit parts in percentage (w/w) were: 71% of pulp, 17% of peel and 12% of seed.
The fruit maturation analyses were performed only for the edible portion of the fruit (peel+pulp). The result for total acidity was 13.21 ± 0.25 % (w/w) and for Total Titratable Acidity (citric acid content) it was 0.92 ± 0.02 % (w/w); the relationship STT/ATT (ratio) was found: 14.13 ± 0.25 and the SST was 13.07 ± 0.12°Brix.
Moisture analysis showed an average of 88.4 ± 0.7% (w/w) of water for the peel; 90.7 ± 0.6% (w/w) for the pulp and 49.9 ± 1.0 % (w/w) for the seeds of grumixama fruit.
The moisture content in the pulp (90.7%) and peel (88.4%) was higher than that found by Arévalo et al. [17] in fruits of E. brasiliensis, Lam. (83.9 % in the pulp and 77.4 % in peel), but lower than studies conducted in camu-camu pulp (92.7%) [18] and uvaia (94.4%) [19] Fruits that belong to the same family Myrtaceae.
Seeds: The initial result for the carotenoids was 17.38 µg.g-1. However, after injection into the chromatograph, a need for saponification was observed, due to the presence of chlorophyll, which was identified after comparison of the UV/VIS spectra of both peaks with data from the literature [20].
Chlorophyll has an intense band of energy absorption centered at 450 nm, which ends up overestimating the results of the carotenoids. After eliminating all chlorophyll by saponification, the result of the carotenoids was 3.64 µg.g-1. Therefore, the initial value must have been practically all chlorophyll. This result prompted the decision not to carry out the analysis of the remaining replicates. Chromatographic analysis of the saponified extract of the grumixama seeds was carried out and despite the low concentrations; the presence of lutein (0.82 µg.g-1), β-cryptoxanthin (1.40 µg.g-1), α-carotene (0.25 µg.g-1) and β-carotene (0.82 µg.g-1) were found.
Pulp and peel: Saponification of the pulp and peel fractions of the fruit had to be carried out due to the presence of esterified β-cryptoxanthin.
The chromatogram evaluation identified eight carotenoids, both in the pulp and in the fruit peel: violaxanthin, lutein, zeinoxanthin, β-cryptoxanthin, 13-cis β-carotene, α-carotene, β-carotene and 9-cis β-carotene (Figure 2).
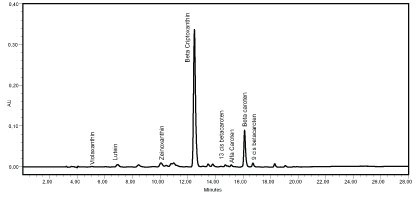
Figure 2: Chromatogram of the carotenoids from grumixama pulp and peel extracts (both had the same carotenoid profile). AU: Absorbance Unit
The analysis was carried out after the saponification step, and the results are shown in table 1.
| Peel | Pulp | |
| Carotenoids | Content (µg.g-1) | Content (µg.g-1) |
| Total | 55.94 ± 1.85 | 26.54 ± 0.62 |
| Violaxanthin | 0.78 ± 0.08 | 0.09 ± 0.02 |
| Lutein | 4.39 ± 0.51 | 0.41 ± 0.01 |
| Zeaxanthin | 1.06 ± 0.11 | 0.59 ± 0.05 |
| β-cryptoxanthin | 39.64 ± 1.35 | 17.47 ± 0.83 |
| α-carotene | 0.17 ± 0.03 | 0.21 ± 0.01 |
| 13 cis β-carotene | 0.79 ± 0.02 | 0.23 ± 0.02 |
| β-carotene | 4.43 ± 0.05 | 4.12 ± 0.28 |
| 9 cis β-carotene | 0.38 ± 0.02 | 0.47 ± 0.06 |
Table 1: Average of total carotenoids in the pulp and peel of the saponified extract (µg.g-1). Results reported are mean values ± SD
The two major carotenoids, β-cryptoxanthin and β-carotene, account for some 71% and 8% of the total carotenoids in grumixama peel, and about 66% and 18% in the pulp of the fruit, respectively. Both are precursors of vitamin A.
Few studies have shown the presence of carotenoids in dark-skinned fruits. This may be due to the dark color of their peels which eventually covers up the characteristic color of carotenoids. However, the separation of anthocyanins during the carotenoid extraction in the water: ether separation step is visible. The purple color remains in the water and carotenoids, which are non-polar, migrate to the ether phase.
The edible part of the grumixama fruit showed about 32.90 µg.g-1 of total carotenoids and 22.27 µg.g-1 of β-cryptoxanthin. In the study carried out by Oliveira et al. [21] about 38 µg.g-1 of β-cryptoxanthin in Formosa papaya was found, which is the main source of carotenoid for this fruit. In order to consider a fruit as a source of carotenoid it must contain at least 20 µg.g-1 of a specific carotenoid [8].
Therefore, grumixama can be considered a new source of β-cryptoxanthin carotenoid, enhancing the value of this fruit as a source of antioxidants and provitamin A, since it is an active carotenoid.
The vitamin C was found in all grumixama fractions: 5.79 ± 0.15 mg.100 g-1 in the peel; 10.55 ± 1.35 mg.100 g-1 in the pulp; and 3.82 ± 0.09 mg.100 g-1 in the seeds.
There are no data in the literature regarding the quantification of ascorbic acid in E. brasiliensis by HPLC. Oranges, which are considered the major source of Vitamin C in traditional Brazilian diet, contain about 60 mg.100 g-1 of vitamin C according to the Brazilian Table of Food Composition [22]. The edible portion of the grumixama fruit has 18.75 mg.100 g-1, approximately 1/3 of this amount. The World Health Organization recommends a daily intake of 45 mg vitamin C [23]; therefore 100 g of the edible portion of the fruit studied corresponds to 41.7% of the RDI (Recommended Daily Intake). Any food that has 10% or more of the daily value of a nutrient is considered a good source of this nutrient; a food that provides 20% of this nutrient is considered rich. Thus, grumixama can be considered rich in vitamin C [24].
Fructose, glucose and sucrose were found in the seeds while only fructose and glucose were found in the peel and pulp of the grumixama fruit.
The results of the sugar content of soluble sugars were as follows: in the pulp, 3.13 ± 0.21% (w/w) of fructose, and 2.84 ± 0.21% (w/w)of glucose; in the peel, 4.18 ± 0.15 % (w/w) of fructose, and 3.68 ± 0.17% (w/w) of glucose and in the seeds,1.13 ± 0.04 % (w/w) of fructose, 1.01 ± 0.04 % (w/w) of glucose and 0.36 ± 0.05 % (w/w) of sucrose.
The total sugar content found in the edible part of the grumixama fruit (7.4%) was slightly higher than found in strawberries (6.8%), but lower than in Surinam cherry (10.2%) according to the Brazilian Table of Food Composition (TACO, 2011) [22].
The fructose concentration was higher than the glucose concentration in all fractions. The presence of reducing sugars (fructose and glucose) is a quality factor for the acceptance of raw or processed fruit [25].
The chromatogram of the extract of freeze dried grumixama peels, presented two major peaks absorbing at 520 nm (Figure 3). The mass spectrum obtained by MS for the anthocyanin 1 (Figure 3) showed a molecular ion with m/z 465 u, referring to delphinidin-3-O-glucoside, and the injection in MS-MS (with increased energy of the Trap and Transfer to 6.0 V) indicated the presence of a glyconedelphinidin with m/z 303 u.
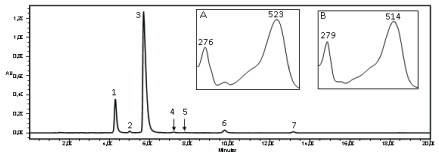
Figure 3: Chromatogram and UV-Vis spectrum of the anthocyanins from grumixama peel extract. Peak 1 and spectrum A: delphinidin-3-O-glucoside; Peak 2: cyanidin-3-O-galactoside; Peak 3 and spectrum B: cyanidin-3-O-glucoside; Peak 4: petunidin-3-O-glucoside; Peak 5: derivative of pelargonidin; Peak 6: peonidin-3-O-glucoside; Peak 7: anthocyanin not identified. AU: Absorbance Unit
Confirmation of anthocyanin 3 (Figure 3) was performed by injection in MS-MS, which showed an ion with m/z 449 u, corresponding to cyanidin- 3-O-glucoside; the increased transfer and trap energies to 6.0 V favored the anthocyanin fragmentation and thus detected cyaniding aglycone with m/z 287 u, due to the loss of a sugar molecule.
Seven anthocyanins were detected in the present study. Among these anthocyanins, three were already reported by Flores et al. [2]: delphinidin- 3-O-glucoside, cyanidin-3-O-galactoside and cyanidin-3-O-glucoside in grumixama fruits.
Anthocyanin 1 was identified as delphinidin-3-O-glucoside (confirmed by comparison with anthocyanin isolated from grape peel extract and by mass spectrometer analysis). Anthocyanin 2 shows the absorption bands λmax 278.3 and 517.7 nm, indicating that it is a cyanidin. This substance was identified as cyanidin-3-O-galactoside, after comparison with Flores et al. [2] study, and also with the absorption spectra UV-Vismax and retention times of anthocyanins obtained from grape peel extracts. Anthocyanin 3 was identified as cyanidin-3-O-glucoside (confirmed by comparison with anthocyanin isolated from grape peel extract and by mass spectrometer analysis). Anthocyanin 4 presented λmax absorption bands 271.2 and 520.1 nm, indicating that the anthocyanin was a petunidin. By comparing absorption spectra UV-Vismax and retention times of anthocyanins from grape peel extracts, the substance was identified as petunidin-3- O-glucoside. Anthocyanin 5 had a maximum absorption band at 499 nm, which characterizes it as a derivative of pelargonidin. Pelargonidin absorption band is the only one next to 500 nm, which was reported in the literature by Hong et al. [26]. Anthocyanin 6 showed maximum absorption at 280.7 and 516.5 nm, indicating that the anthocyanin was a peonidin. After comparison with the absorption spectra UV-Vismax and retention times of anthocyanins from grape peel extracts, the substance was identified as peonidin-3-O-glucoside. The seventh anthocyanin was not identified. The concentration of anthocyanins detected is showed in table 2.
| Anthocyanins (mg.100 g-1) | |||||||
| Dp-3-glu | Cy-3-galac | Cy-3-glu | Pt-3-glu | Pg derivate | Pn-3-glu | ACN 7 | Total monomeric ACN |
| 949.06 ± 20.13 | 27.89 ± 0.77 | 3729.50 ± 72.75 | 22.02 ± 1.06 | 9.09 ± 0.43 | 91.36 ± 1.30 | 8.30 ± 1.34 | 4837.21 ± 91.43 |
Table 2: Concentration of anthocyanins (mg.100 g-1) found in freeze-dried grumixama peel. Results reported are mean values ± SD
Dp-3-glu: delphinidin-3-O-glucoside; Cy-3-galac: cyanidin-3-O-galactoside; Cy-3-glu: cyanidin-3-O-glucoside; Pt-3-glu: petunidin-3-O-glucoside; Pgderivate: pelargonidinderivate; ACN: anthocyanin
The method [14] is specific for monomeric anthocyanins. The two major anthocyanins were equivalent to more than 96% of the total anthocyanins.
The main anthocyanins identified, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, are the same present in major concentration in jabuticaba (Myrciariacauliflora, Berg) peel, which is a fruit with interesting functional potential. According to Lequinste et al. [27] the anthocyanins present in jabuticaba peel showed great antioxidant effects and health benefits, such as on obesity and on insulin resistance.
Freeze dried grumixama peel showed a much higher content of anthocyanins (4837.2 mg.100 g-1) than found by Leite-Legatti et al. (2012) [28] for jabuticaba freeze dried peel (2598.32 mg.100g-1), which is considered rich in this phenolic compound. Thus, grumixama can be considered a new source of anthocyanins, adding value to this fruit that is still not commercially exploited.
The free phenolic compounds identified were: catechin, ellagic acid, rutin, isoquercetin, quercetin in the peel and pulp and gallic acid, ellagic acid, syringic acid, rutin, catechin and p-hydroxybenzoic acid in the grumixama seed.
The hydrolysis of the samples promoted a more complete characterization of the phenolic compounds. Following the hydrolysis procedure gallic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, ellagic acid, rutin and isoquercetin were identified in the fruit peel; gallic acid, protocatechuic acid, p-hydroxybenzoic acid, ellagic acid, and rutin in the pulp; and gallic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic, and ellagic acid in the seeds. Figure 4 shows all the phenolic compounds identified in the grumixama peel.
The identification of flavonoids and phenolic acids was performed by comparison with commercial standard retention times and analyzing the absorption spectrum at 270 nm, 310 nm and 370 nm. Isoquercetin was not quantified due to the unavailability of sufficient amount of mass for a standard solution preparation. The concentrations obtained are shown in table 3.
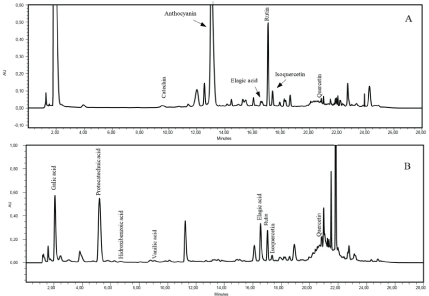
Figure 4: a) Chromatogram of phenolic compounds from Grumixama peel before hydrolysis; b) Chromatogram of phenolic compounds from Grumixama peel after hydrolysis
| Fruit Fractions | Phenolic compound concentrations (mg. g-1) | ||||||||
| Catechin | Rutin | Quercetin | Protocatechuic acid | 4-hydroxybenzoic acid | Vanillic acid | Gallic acid | Ellagic acid | Syringic acid | |
| Peel (free fraction) | 0.3210 ± 0.09 | 2.0721 ± 0.02 | 0.0171 ± 0.00 | ND | ND | ND | ND | 0.1906 ± 0.01 | ND |
| Peel (hydrolysate) | ND | 0.1629 ± 0.04 | 0.0287 ± 0.00 | 0.0496 ± 0.00 | 0.0065 ± 0.00 | 0.0067 ± 0.00 | 0.195 ± 0.01 | 0.0573 ± 0.00 | ND |
| Pulp (hydrolysate) | ND | 0.0044 ± 0.00 | 0.0110 ± 0.00 | 0.0115 ± 0.00 | 0.0069 ± 0.00 | 0.0098 ± 0.00 | 0.1451 ± 0.01 | 0.2670 ± 0.02 | ND |
| Pulp (free fraction) | 0.0288 ± 0.00 | 0.0644 ± 0.01 | 0.0046 ± 0.0002 | ND | ND | ND | ND | 0.0468 ± 0.00 | ND |
| Seed (hydrolysate) | ND | ND | ND | 0.0115 ± 0.00 | 0.0188 ± 0.00 | 0.0168 ± 0.00 | 1.2208 ± 0.1588 | 1.3185 ± 0.16 | ND |
| Seed (free fraction) | 0.2408 ± 0.01 | 0.1652 ± 0.00 | ND | 0.1115 ± 0.00 | 0.0483 ± 0.00 | ND | ND | 0.2736 ± 0.01 | 0.0173 ± 0.00 |
Table 3: Concentration of phenolic compounds found in grumixama (mg.g-1). Results reported are mean values ± SD
The value for ellagic acid (0.26 mg.g-1) was the same to the one found by Reynertson et al. [29] (0.26 mg.g-1), however, the quercetin concentration (0.019 mg.g-1) was lower than the one obtained by the same authors (0.04 mg.g-1). Alsorutin was found by Reynertson et al. [29] (0.08 mg.g-1) but it was inferior to the value (0.49 mg.g-1) found in the edible part of grumixama.
Besides the above characterized phenolic compounds, there were 39 peaks detected but not identified in the pulp, 31 unidentified peaks in the seeds and 45 unidentified peaks in the peel of grumixama. All these peak absorption spectra UV-Vismax showed characteristics of phenolic absorption bands; however it was not possible to identify them due to the absence of analytical standards. The phenolic compounds mainly occur as soluble and insoluble conjugates, covalently bound to the sugars or structural components of the cell wall [30]. The identification of these substances may be possible by isolating these and using other techniques such as mass spectrometry and NMR in an attempt to elucidate the structures of these compounds.
Grumixama fruits show great diversity and high concentrations of bioactive compounds, such as anthocyanins, other flavonoids, phenolic acids, carotenoids and vitamin C. The peel of E. brasiliensis, Lam. was the richest part of the fruit and presents a potential to be used in the food industry, as an additive or natural colorant in products that can promote possible health benefits.
Based on the results, E. brasiliensis, Lam. can be considered an excellent food with potential functional properties. Its composition should stimulate the production and consumption of this under-exploited Brazilian fruit.
Download Provisional PDF Here
Article Type: Research Article
Citation: Nascimento LSM, Santiago MCPA, Oliveira EMM, Borguini RG, Braga ECO, et al. (2017) Characterization of Bioactive Compounds in Eugenia brasiliensis, Lam. (Grumixama). Nutr Food Technol Open Access 3(3): doi http://dx.doi. org/10.16966/2470-6086.146
Copyright: © 2017 Nascimento LSM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access