Introduction
Whey protein concentrates (WPC) and isolates (WPI) are important food ingredients because of their desirable functional properties, such as gelation, foaming, and emulsification. Whey proteins are a significant source of functional protein ingredients for many traditional or novel food products [1]. The main proteins in whey are β-lactoglobulin (β-lg), α-lactalbumin (α-lac) and bovine serum albumin (BSA) and they account for 70% of total whey proteins [2]. These proteins are responsible for the functional properties of whey proteins, such as solubility in water, viscosity, gelation, emulsification, foaming, colour, and flavor and texture enhancement and offer numerous nutritional advantages to formulated products.
Proteins due to their amphiphilic character can adsorb at fluid interfaces. The adsorption of proteins at interfaces and other dynamic surface properties-such as film viscoelasticity-are known to play an important role in the formation and stability of food dispersed systems as foams and emulsions [1]. Because of the adsorption process, protein molecules prevent the recoalescence of previously created bubbles or droplets. In addition, during the protein adsorption the surface or interfacial tension at the air–water or/and oil–water interface decreases which is an important attribute to optimize the input of energy involved in the foaming or emulsification process [2]. Smaller bubbles or droplets are of interest as an important factor for the stability of colloidal systems.
A structure-function relationship of whey proteins has been widely studied in literature, especially as it relates to their aggregative properties and nature of interactions (e.g., hydrophobic interactions, hydrogen bonding, electrostatic interactions and thiol-disulfide exchange reactions) [2]. These interactions can be tailored by altering the physicochemical properties of the whey protein molecules by pre-treating the proteins with temperature to partial or completely unravel the protein structure to expose buried hydrophobic moieties [3].
Polysaccharides are used in admixture to proteins mainly to enhance stability of dispersed systems. Most high-molecular weight polysaccharides, being hydrophilic, do not have much of tendency to adsorb at the air-water interface, but they can strongly enhance the stability of protein foams by acting as thickening or gelling agents [4]. Hydroxypropylmethylcellulose (HPMC) applications are based in the methyl substitutions that constitute hydrophobic zones along the cellulose backbone, whereas hydroxypropyl groups are more hydrophilic. The introduction of these hydrophobic groups allows HPMC to behave as a surfactant. Thus HPMCs are adsorbed at fluid interfaces lowering the surface tension [3-5]. HPMC is a surfaceactive cellulose derivative, that is used in the food industry to improve the quality of baked products [6] and the pharmaceutical industries in controlled drug-release matrixes [7,8].
The surface pressure isotherms, the structural and surface dilatational properties and the dynamics of adsorption of three commercial types of HPMC (E4M, E50LV and F4M) adsorbed films at the air–water interface were previously studied [9,10]. In these works we have concluded that HPMC molecules are able to diffuse and saturate the air–water interface at very low concentrations in the bulk phase. The three HPMCs formed very elastic films at the air–water interface, even at low surface pressures.
E4M showed a distinct behaviour in comparison with other celluloses as it showed a competitive behaviour at all bulk concentrations. The strong competitive behaviour of E4M should be attributed to its higher surface activity that arises from its molecular structure. E4M have hydroxypropyl molar substitution (MS)=0.23, which is the highest of its series. The hydroxypropyl groups are more hydrophilic than methyl groups and more likely to form hydrogen bonds to the water molecules as determined by NMR [11]. Nevertheless, both the methyl and the hydroxypropyl groups render the cellulose hydrophobic [12]. Another feature of E4M, should be attributed to the higher molecular weight of E4M which allow to this polysaccharide its strong competence capacity. The surface tension decrease is not dependent on the molar adsorption of the polymer, but it depends on the number of polymer segments, which are in actual contact with the surface [12]. This means that the surface properties of a polymer depend on the length and distribution of trains, loops and tails. The average degree of polymerization of E4M was higher than E50LV (data supplied by Dow Chemical Co), which involves an increase in the number of segments that potentially could be adsorbed per mol of polymer [13].
When WPC adsorbs at the air-water interface in the presence of E4M three phenomena can occur: (i) the polysaccharide adsorbs at the interface on its own in competence with the protein for the interface (competitive adsorption) (ii) the polysaccharide complexates with the adsorbed protein mainly by electrostatic interactions or hydrogen bonding, and (iii) because of the existence of a limited thermodynamic compatibility between the protein and polysaccharide, the polysaccharide concentrates the adsorbed protein.
Aggregation phenomena could be enhanced upon heating with aggregates formation as a consequence. It is evident that surface pressure of WPC / E4M mixed systems cannot be easily predicted because complex phenomena are occurring simultaneously:
- Competitive or cooperative behaviour as a function of each biopolymer bulk concentration and molecular characteristics of E4M;
- Incompatibility between the biopolymers at the bulk and also at the interfacial level.
The interactions or incompatibilities between proteins and polysaccharides influence the rate and the magnitude of adsorption. Therefore, the order in which the different components get the interface will influence the final equilibrium surface composition [14].
The study of competitive adsorption of surface-active proteins and polysaccharides attracts interest because of the potential synergism of mixed biopolymers at fluid interfaces [15,16]. In those works, we conclude that due to their surface-active character, competitive adsorption could occur in mixtures of these polysaccharides and WPC proteins. In Perez, et al. [14] the competitive behaviour impact of HPMC on the WPC adsorbed films at the air–water interface and on their rheology were studied [10,17- 18]. Recently, we analyzed the structural and rheological properties on the spread WPC monolayer at the air-water interface influenced by the three previously characterized HPMCs [4]. As E4M showed the highest interfacial tension-activity between the studied HPMCs in the previous work, we decided to use at present. The combination of methods used to reach the objectives originally proposed for this project would get complementary information, which in turn could be applied with technological ends. In fact, the application of mixing biopolymer films could help us to get inside in the stabilization of emulsions and foams.
In this context, the objective of this work was to determine the effect of heat application on whey protein concentrate (WPC) in combinations with hydroxypropylmethylcellulose commercially named E4M, to study the foaming properties in relation with interfacial ones.
Materials and Methods
Samples preparation and heat treatment
WPC powder was kindly supplied by Milka Frank, Santa Fe, Argentina. Its composition was: proteins 78.9% (N × 6.25) lactose 5%, ash 4.3% and moisture 5.6%. WPC PAGE-electrophoresis in native conditions was made in a Mini-Protean II dual slab cell system (Bio-Rad Laboratories). Quantification of the protein bands was accomplished by means of BioRad GS-670 imaging densitometry. Bio-Rad Molecular Analyst/PC. Molecular Image program allowed the analysis of molecular weight and band intensities under volumetric test option. WPC composition was: β-lg 44%, α-lac 20.1%, BSA 8%. The remainder proteins constituting the minor fraction were immunoglobulins and the proteose-peptone fraction [19].
HPMC (Methocell serie of E4M), food grade, from The Dow Chemical Company were kindly supplied by Colorcon-Argentina and used without purification. The more relevant physicochemical properties of E4M are: methyl and hydroxypropyl content: 28 and 10.2%; methyl/hydroxypropyl ratio: 2.7; degree of substitution: 2.13; viscosity (20°C) of 2% wt solution: 4965 cp; and molecular weight: 90000 kDa.
The pH and the ionic strength (0.05M) were kept constant in all the experiments by dissolving the biopolymers in Trizma buffer solution [(CH2 OH)3 CNH2 /(CH2 OH3 CNH3 Cl) (Sigma, >99.5%). Milli-Q ultrapure water pH 7 was always used. Solutions were kept 12 h at 4°C to achieve the maximum biopolymer hydration.
The WPC/E4M mixed systems were obtained by mixing the appropriate volume of each double concentrated biopolymer solution up to achieve the required final concentration.
The following mixed systems were studied: WPC 1 × 10-2%+E4M 1%; WPC 1%+E4M 1 × 10-2% and WPC 1%+E4M 1% w/w. Single WPC or E4M solutions at the same concentration into mixture were used as experimental controls.
Thermal treatment of solutions was performed by heating 50 ml of the respective solution into glass flasks with hermetic seal. Flasks were immersed in a temperature controlled bath at 90°C for 30 min.
Foam formation and stability measurement
Determinations of foam formation and stability were performed using a Foamscan instrument (Teclis-It Concept, Logessaigne, France). The foam is generated by blowing nitrogen gas at a flow of 45 mL/min through a porous glass filter of 0.2 µm at the button of a glass tube where 20 ml of the foaming aqueous solutions (25 ± 1°C) is placed. In all experiments, the foam was allowed to reach a volume of 120 ml. The bubbling was then stopped and the evolution of the foam was analyzed by means of conductimetric and optical measurements.
Four parameters were determined as a measure of foaming capacity. The overall foaming capacity (OFC, ml/s) was determined from the slope of foam volume curve till the end of the bubbling, which indicates a general foamability of the system. The foam capacity (FC), a measure of gas retention in the foam, was determined by Equation 1. The foam maximum density (MD), a measure of the liquid retention in the foam, was determined by Equation 2.The relative foam conductivity (Cf, %) is a measure of the foam density and was determined by Equation 3.
\[{\rm{FC = Vfoam}}({\rm{f}}){\rm{/Vgas}}({\rm{f}})\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,({\rm{1}})\]
\[{\rm{MD = Vliq(i) - Vliq(f) / Vfoam(f)}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,({\rm{2)}}\]
\[{\rm{CF = Cfoam(f) /Cliq(f) }}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(3)\]
Where Vfoam (f) is the final foam volume, Vgas (f) is the final gas volume injected, Vliq (i) and Vliq (f) are the initial and final liquid volumes, and Cfoam (f) and Cliq (f) are the final foam and liquid conductivity values, respectively. The static foam stability was determined from the volume of liquid drained from the foam over time [20]. The half-life time (t1/2), referring to the time needed to drain V/2 can be expressed by Equation 4 corresponding to an empirical second order equation.
\[{{\rm{t}}_{{\rm{1/2}}}}{\rm{ = (}}{{\rm{k}}_{\rm{2}}}{\rm{ }}{{\rm{V}}_{\rm{0}}}{\rm{ }}{{\rm{)}}^{{\rm{ - 1}}}}{\rm{ }}\,\,\,\,\,\,\,\,\,\,\,{\rm{(4)}}\]
Dynamic surface tension
Time-dependent surface pressure (π) of adsorbed WPC/E4M mixed films at the air–water interface was performed by an automatic drop tensiometer as described elsewhere [21]. Briefly, aqueous solutions were allowed to stand for 30 min to reach constant temperature in the compartment. Then a solution drop was delivered and allowed to stand at the syringe tip for about 180 min to achieve adsorption at the air–water interface. The image of the drop was continuously taken from a CCD camera and digitalized. The surface tension (ρ) was calculated through the analysis of the drop profile [22]. The surface pressure is π=ρo -ρ, where ρo is the surface tension of pure solvent in the absence of macromolecules. The average accuracy of the surface tension was roughly 0.1 mN/m. However, the reproducibility of the results (for at least two measurements) was better than 1%.
Kinetics of adsorption
The kinetics of protein/polysaccharide adsorption at the air–water interface can be monitored by measuring changes in surface pressure.
The adsorption of these biopolymers at a fluid interface includes (i) the diffusion of the protein from the bulk onto the interface, (ii) adsorption (penetration) and interfacial unfolding, and (iii) aggregation (rearrangement) within the interfacial layer, multilayer formation and even interfacial gelation.
During the first step, at relatively low surface pressures, when diffusion is the rate-determining step, a modified form of the Ward and Tordai equation [23] can be used to correlate the change in surface pressure with time Equation 5.
\[\pi = {\rm{ }}2{C_0}{\rm{ }}KT{\left( {Dt/{\rm{ }}3.14} \right)^{1/2}}{\rm{ }}{K_d}{t^{1/2}}\,\,\,\,\,\,\,\,\,\,\,\,(5)\]
Where C0 is the concentration in the bulk phase, K is the Boltzmann constant, T is the absolute temperature, and D is the diffusion coefficient.
If the diffusion of the biopolymer at the air–water interface controls the adsorption process, a plot of π versus t1/2 will then be linear [24-28], and the slope of this plot will be the diffusion rate constant (kd ). At higher adsorption time, in the period after that affected by the diffusion, an energy barrier for WPC/E4M adsorption exists, which can be attributed to adsorption, penetration, unfolding and rearrangements of the macromolecules at the interface [29]. Because the interfacial concentration of adsorbed macromolecules is several times higher than that in the bulk phase, molecular unfolding and rearrangement steps are magnified processes happening at interface. To monitor adsorption/penetration/ unfolding of adsorbed WPC/E4M molecules, the approach proposed by Graham and Phillips [27] was used. Thus, the rate of these processes can be analyzed by a first-order equation:
Where π180, π0 and πt are the surface pressures at 180 min of adsorption time, at time t=0, and at any time t, respectively, and ki is the first-order rate constant. In practice, a plot of Equation 6 usually yields two or more linear regions. The initial slope is taken to correspond to a first-order rate constant of adsorption (Kp ), while the second slope is taken to correspond to a first-order rate constant of rearrangement (Kr ), occurring among a more or less constant number of adsorbed molecules. All measures were made at least two times and errors less of 10% were obtained.
\[In\left( {{\pi _{180}}{\rm{ - }}{\pi _t}} \right)/({\pi _{180}}{\rm{ - }}{\pi _0}{\rm{) = - }}{k_{it}}\,\,\,\,\,\,\,\,\,\,\,(6)\]
Surface dilatational properties
Time dependent surface viscoelastic parameters of adsorbed films at the air-water interface were performed by an automatic drop tensiometer (IT Concept, France) as described elsewhere [18,22]. Surface dilatational modulus, E, and its elastic, Ed, and viscous, Ev, components, were measured as a function of time, θ at 15 % deformation amplitude (ΔA/A) and at 100 mHz of angular frequency (ω). The percentage area change was determined to be in the linear region (data not shown). The method involved a periodic automated-controlled, sinusoidal interfacial compression and expansion performed by decreasing and increasing the drop volume, at the desired amplitude. The surface dilatational modulus derived from the change in surface tension (dilatational stress), σ Equation 7, resulting from a small change in surface area (dilatational strain), A (Equation 8), may be described by Equation 9 [20]
\[\sigma = {\sigma _0}{\rm{ }}sin\left( {\omega \theta + {\rm{ }}\delta } \right){\rm{ }}\,\,\,\,\,\,\,{\rm{ }}\left( 7 \right)\]
\[A = {A_0}sin\left( {\omega \theta } \right)\,\,\,\,\,\,\,\,\,\,{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} \left( 8 \right)\]
\[E = \frac{{d\sigma }}{{dA/A}} = \frac{{d\pi }}{{dInA}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(9)\]
Where σ0 and A0 are the stress and strain amplitudes, respectively, and δ is the phase angle between stress and strain.
The dilatational modulus is a complex quantity, which is composed of real and imaginary parts (Equation 10).
\[E = \left( {\sigma /{\rm{ }}{A_0}} \right)\left( {cos\delta + isin{\rm{ }}\delta } \right){\rm{ = }}{E_d} + i{E_v}{\rm{ }}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\left( {10} \right)\]
The real part of the dilatational modulus or storage component is the dilatational elasticity, Ed = E cosδ . The imaginary part of the dilatational modulus or loss component is the surface dilatational viscosity Ev = E senδ . The ratio (σ0 /A0 ) is the absolute modulus, │E│, a measure of the total unit material dilatational resistance to deformation (elastic + viscous). For a perfectly elastic material, the stress and strain are in phase (δ=0) and the imaginary term is zero. In the case of a perfectly viscous material δ=90º and the real part is zero. The loss angle tangent (tan δ) can be defined by Equation 11. Thus, if the film is purely elastic, the loss angle tangent is zero.
\[tan\delta = {\rm{ }}{E_v}{\rm{ }}/{\rm{ }}{E_d}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(11)\]
The experiments were carried out at 20°C and the temperature of the system was maintained constant within ± 0.1°C by circulating water from a thermostat. Samples solutions were placed in the syringe, which in turn was placed into the compartment. Finally, the samples were allowed to stand for 30 min to reach the desired constant temperature. Then a drop of sample solution was delivered to achieve macromolecule adsorption at the air-water interface. The materials in contact with solutions under examination were properly cleaned in order to avoid any contamination by any surface-active substance.
Statistical analysis
All the experiments were performed at least in duplicate. The model goodness-of-fit was evaluated by the coefficient of determination (R2 ) and the analysis of variance (ANOVA), using Statgraphics Plus 3.0. software. Manigistics, Inc 2115 East Jefferson Street, Rockville, Md 20876, USA.
Results and Discussion
Foaming characteristics
First of all, it is crucial to mention that stable foam cannot be obtained from single WPC dissolved in buffer, irrespective of the concentrations used. That is, under this condition, WPC does not foam enough to reach 120 mL of foam because the rate of formation and stabilization of new bubbles is lower than the rate of foam rupture. This is in line with previous reports [24,25]. Meanwhile, simple E4M solutions foamed concentrations of 1% w/w or higher in combination with WPC. However, the lowest concentration (1 × 10-2% w/w) of E4M foamed when was combined with high protein concentration.
Foaming capacity: The overall foaming capacity (OFC, ml/s), the FC, the foam MD, and the relative foam conductivity (Cf, %) for samples WPC 1 × 10-2%+E4M 1%; WPC 1%+E4M 1 × 10-2% and WPC 1%+E4M 1% are shown in Figure 1(a-d).
Only for one mixed system, WPC 1% + E4M 1×10-2% heat treatment improved the OFC, FC, MD and Cf %. At the highest E4M concentration such as that containing E4M 1% no effects of heat treatment were found, only a little decrease of FC, was observed, a lower gas retention was detected in the foam as a consequence of heat treatment application (Figure 1b).
For WPC 1% + E4M 1 × 10-2% mixed system all measured parameters resulted in a notable increment after heat treatment. The foam formed consisted of denser bubbles (not shown) with high liquid and gas retention in the foam. This result could be attributed to protein heat denaturation favoring its faster diffusion to the air-water interface at short times.
The general parameters and particularly FC presented low value for WPC 1% + E4M 1 × 10-2% system without heat treatment. However, when heat was applied a considerable increment was found. It is important to have in mind that, pure E4M only form foam at a concentration of 1% wt. At lower E4M concentrations it does not foam, whereas, pure WPC solution did not foam at any condition. This result was very remarkable for this mixed system.
In respect to WPC 1% + E4M 1%, where equal proportions of each macromolecule was in solution, no effect of heat treatment was observed. E4M has a superior diffusion velocity than WPC at liquid interface [28], which improve foamability [29]. This feature would be contributing to enhance the performance of foaming parameters during foam formation (time<60 s) which were not modified by heat treatment with a exception of WPC at 1% + E4M at 1 × 10-2%, which was a for general low viscosity system.
Foam stability: The static foam stability was determined from the volume of liquid drained from the foam over time. The half-life time, (tt1/2 drain), was obtained from the experimental data Equation 4. Figure 1e shows the foam stability quantified as the half-life time for WPC 1 × 10-2% + E4M 1%; WPC 1% + E4M 1 × 10-2% and WPC 1% + E4M 1% systems.
The maximum stability obtained was observed for untreated WPC 1 × 10-2% + E4M 1%, it means; at the lower protein concentrations in the mixed solution, the foam drainage stability was the highest. In the mixed system, components interaction would promote a complex interfacial film formation, stabilized mainly by E4M with a proved higher surface pressure than WPC [28]. Results obtained there also indicate that WPC and E4M competed by the air-water interface at short adsorption times. The competition increased with time. E4M molecules penetrate and rearrange into the monolayer, and surface pressure increased in a higher extent that WPC penetration occurs [14]. This fact reflects the E4M greater capacity of molecular reorganization. Thus, WPC 1×10-2% + E4M 1% system resulted in the highest stability whereas for WPC 1% + E4M 1% the probability of competence between absorbing biopolymers increased.
For a particular protein the overall foam destabilization (the half-life time of the foam) and the individual destabilization processes (drainage, disproportionation and coalescence) may be related to the interfacial characteristics (protein concentration and aggregation at the interface, structure, topography, and interfacial shear and dilatational characteristics) of the protein film adsorbed around the bubbles [30]. All processing techniques as heating treatment would alter the interfacial structure and consequently change their functional properties. [31].
Drainage, collapse and disproportionation which are mechanisms of foam instability related to protein-protein and protein aqueous phase interactions, protein denaturation at higher temperatures has to be taken into account. Protein denaturation induced by heat could increase the protein-protein interactions. This fact could produce aggregation and even precipitation [32] and would reduce the surface activity of the protein causing a stability decrease of WPC 1 × 10-2% + E4M 1% treated system. However, when content of protein was higher, (WPC 1% + E4M 1%), protein and polysaccharide mutually exclude one another, segregating into different phases. Excluded volume effects provoked faster association of macromolecules and enhancement of protein adsorption al fluid interfaces [33]. Heat denaturation allows to display hydrophobic groups transforming WPC 1% + E4M 1% heat treated solution in a more surface active system leading to more stable foam against drainage of liquid.
Dynamics of adsorption
As described before, WPC showed surface activity from bulk concentrations as low as 5 × 10-5 [18], even though it does not foam. Upon biopolymer bulk concentration, the adsorption process describes the following steps: a) at low solution concentration the more hydrophobic residues take place at the interface, surface pressure is practically zero since the number of adsorbed segments is not enough to cause a significant decrease in surface tension; b) at higher biopolymer concentration the formation of an adsorbed monolayer occurred, which has an expanded structure, the Structure I; c) as the bulk, and interfacial concentration increases Structure I suffer a transition to structure II, an equilibrium situation can be established when the monolayer is saturated by the irreversible adsorbed biopolymer segments; d) finally, the formed film can collapse with multilayer formation at the highest bulk concentration [18].
Having into account the results obtained for the foaming assay for mixed systems, surface pressure upon short and long times, was studied by an automatic drop tensiometer. π-time curves for single components were included in the figures in order to facilitate data interpretation (Figures 2a-f). WPC 1 × 10-2% + E4M1% was evaluated in first place. It can be seen that mixture showed higher surface activity than the single protein at short adsorption times (t<60s). Under such conditions, WPC bulk concentration is not enough to saturate the interface (structure II), meanwhile E4M can do it (Figure 2a). Surface pressure immediately increased after drop formation, which obey to the polysaccharide adsorption, as can be seen in Figure 2a, corresponding to short adsorption times [14]. A slight difference could be detected in this mixed system, which showed a decrease in π values at times <60s, after heating.
At long adsorption times (t>10000s), mixture and heated mixture reached π values similar to single protein (Figure 2b).
When the protein concentration increased, as in the WPC 1%/E4M 1×10-2%, the opposite behavior was observed. Under this condition protein can saturate the interface almost instantaneously (structure II), determining the surface pressure of mixed system, at (t <60s) (Figure 2c). E4M presence and heating provoked a slight increase for surface pressure up to 60s (Figure 2c), even a strong synergistic effect was observed at the longest times (Figure 2d). A similar effect could be observed at short adsorption times when the biopolymers concentration was WPC 1%/ E4M 1%. Mixed systems showed an increased surface activity, at both short (Figure 2e) and long (Figure 2f) adsorption times. Even though heated samples did not manifest differences with unheated mixtures. The competitive behavior of E4M has been attributed to its higher surface activity, a consequence of its molecular structure [18]. Surface tension decrease is not dependent on the molar adsorption of the polymer, but it depends on the number of potentially adsorbed segments, that are in contact with the surface [12]. In other words, the adsorption of a polymer depends on the length and distribution of trains, loops and tails. In fact, E4M has a high polymerization degree which increases the number of segments that potentially could be adsorbed per mol of polymer [13].
Kinetics of adsorption
Diffussion stage controls the protein and polysaccharides adsorption process at short times [34]. Thus, from the slope of the plot of π against t1/2 it was deduced the diffusion rate (Kd ) of macromolecules towards the interface. The π-t1/2 plots showed that systems an aqueous phase diffusion step was too fast to be detected by the experimental technique used in this work (π>10 mN/m). However, for these systems the slope of the π-t1/2 curve at the beginning of the adsorption (at 0.5 s) can be considered as a measure of the apparent rate of diffusion, Kd . [35]. Kinetics parameters described from adsorption process Kd , Kp and Kr are shown in the Table 1
| Mixed system |
Kd*(mN·m-1·s-0.5) |
HKd* (mN·m-1·s-0.5) |
Kp × 104 (s-1) |
H K × 104 (s-1)
p |
Kr × 104 (s-1) |
HKr × 104 (s-1) |
| WPC 1 × 10-2%+E4M 1% |
90.83 |
81.43 |
3.45 |
4.67 |
2.96 |
2.77 |
| WPC 1%+ E4M 1 × 10-2 |
58.88 |
70.14 |
4.06 |
4.16 |
7.34 |
5.29 |
| WPC 1%+E4M 1 % |
82.65 |
84.55 |
3.33 |
5.63 |
5.21 |
3.63 |
Table1: Kd (diffusion), Kp (penetration) and Kr (rearrangement) velocities for untreated and heat treated mixed systems.
*The diffusion step is too fast to be detected by the experimental technique used in this work (π>10 mN/m)
The highest Kd were obtained when E4M were in the highest concentration, because of its faster diffusion rate times <60s, and as consequence of higher surface activity and it high bulk concentration. However, Kd of WPC 1% + E4M 1 × 10-20/0% was the unique system with a great increase upon heating (from 58.88 to 70.14 mN·m-1s-0.5).
No differences were observed for the other mixed systems. Results were obtained for foaming parameters (Figure 1a-d) which were previously related to macromolecules diffusion to the air-water interface at short times [36]. Protein heat denaturation or even aggregates formed could favor its diffusion as was stated in the foaming parameters section.
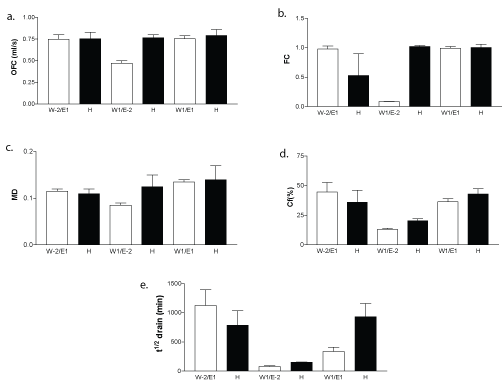
Figure 1: WPC 1 × 10-20/0%+E4M 1%; WPC 1%+E4M 1 × 10-20/0% and WPC 1%+E4M 1 % untreated and heat (H) treated for Overall Foaming Capacity (OFC) (a), Foaming Capacity (FC) (b), foam maximum density (MD) (c), relative foam conductivity (Cf%) (d) And the half-life time for drainage (t1/2 drain) (e).
The corresponding increases of π values in the beginning of adsorption (Figure 2) correspond to the quantity of macromolecules adsorbed at short times [37]. No differences were observed between the unheated systems. In other hand, when heat was applied, over WPC 1% + E4M 1 % mixed system an increment of Kp was found (from 3.33 to 5.63.10-4 s-1). Apparently, when protein denaturation at high proportion took place by heating, E4M penetration was facilitated, at (t<10000s). In a previous publication [38] showed that, E4M 0.25%wt/wt presented the highest penetration rate, which decreased when soy proteins were present at 2%. It means that by comparing separately, E4M had a better ability to penetrate to the interface, but when both biopolymers were together, interactions between them would promote different performance on dynamics measurements. It was seen under these conditions, an increase of rates was observed due to a faster diffusion of proteins to the interface, phase separation (i.e aggregation of the protein induced by the polysaccharide) and increase of surface hydrophobicity by the unfolding of protein molecules [39,40]. Heat treatment could even favor protein-protein interaction, inducing aggregates formation which in turn promoted the E4M penetration in a higher extent.
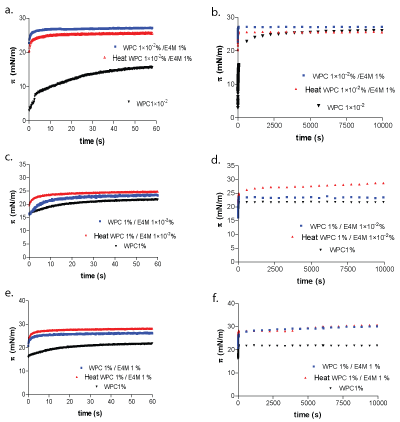
Figure 2: π vs. time for single WPC (close triangle), for untreated (square) and heat treated (triangle) mixed systems of WPC 1 × 10-20/0%+E4M 1% (a), WPC 1%+E4M 1 × 10-20/0% (b), WPC 1%+E4M 1 % (c).
It is supposed that at long times of adsorption, rearranges of molecules at liquid interfaces would be relationed with stability of foams. However, when rearrangement rate for mixed systems were analyzed, an inverse tendency was obtained respect to drainage times. It was observed that the lowest Kp (WPC 1 × 10-20/0%+E4M 1%) resulted in a better t1/2 drain, obtaining the same results for heated samples. No clear tendence was observed for Kr parameter.
Table 2 also shows the surface pressure at 180 min (π180 min) for untreated and heat treated mixed systems. First of all it should be notice that the surface pressure at long times of adsorption can be relationed with t1/2 drain of foams (Figure 1e) such a relation was previously established by [41]. It can be observed that mixed systems with high protein concentration, WPC 1%+E4M 1 × 10-2% and WPC 1%+E4M 1% (protein film structure II) presented the lowest surface pressure at long times and in turn had low t1/2 drain. When heat treatment was applied, an increment of surface pressure at long adsorption times was observed as same way as t1/2 drain (Figure 1e). Thus, it can be said that E4M acts as the determining agent for foams formation, whereas, whey proteins seems to be fundamental in the stability of mixed systems of foams, incrementing the liquid interface strength after heat application. While protein heat denaturation would increment protein-protein interactions at short times, such aggregates would lead to better film formation at long adsorption times, increasing surface pressure and giving higher foams stability.
| Mixed system* |
π180min(mN/m) |
Hπ180min (mN/m) |
Ed180min (mN/m) |
HEd180min (mN/m) |
Tg δ180min |
HTg δ180min |
| WPC 1 × 10-2%+E4M 1% |
26.77 ± 0.1 |
25.30 ± 0.1 |
16.36 |
13.77 |
0.42 |
0.66 |
| WPC 1%+E4M 1 × 10-2 |
21.46 ± 0.1 |
23.73 ± 0.1 |
47.52 |
56.45 |
0.26 |
0.20 |
| WPC 1%+E4M 1 % |
25.48 ± 0.1 |
27.42 ± 0.1 |
47.24 |
49.19 |
0.34 |
0.43 |
Table 2: Surface pressure at 180 min of adsorption time (π180 min) Ed and tan δ for untreated and heat treated mixed systems.
*Mean ± SD; n= 3; H=heated samples
Surface dilatational elasticity of adsorbed films
Values of surface dilatational modulus (E) were similar to the corresponding for Ed, which reflects elastic character of WPC films. It can be seen from Figure 3 that WPC solution (1 × 10-2%, wt) (structure I) showed a maximum Ed value and a further decrease with time. In fact, Ed duplicated their equilibrium values when bulk protein concentration increase from 1×10-2 up to 1%, w/w (Figure 3a-c). This effect was attributed to the highest amount of protein reaching the air-water interface as adsorption time elapsed [28]. Such a decrease may indicate slow structural change due to protein unfolding occurring at the air-water interface [17]. The protein film adopted a less condensed structure, which could explain the Ed decrease after 500s. E4M bulk concentration was higher than that of the protein in mixed system constituted by WPC 1 × 10-2%+E4M 1%wt, under such conditions the diffusion of the polysaccharide molecules would be favoured [10]. Therefore, the solid character was more influenced by E4M, even at long term of adsorption. When protein bulk concentration was not enough to saturate the interface, protein can form films with a higher solid character than E4M. A slight decrease in Ed values was obtained for heated samples.
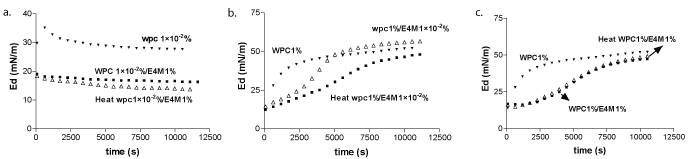
Figure 3: Ed vs. time for alone WPC (close triangle), for untreated (square) and heat treated (triangle) mixed systems of WPC 1 × 10-20/0%+E4M 1% (a), WPC 1%+E4M 1 × 10-20/0% (b), WPC 1%+E4M 1 % (c).
Ed behavior can be described as sigmoidal for WPC 1%, which implies that the solid like character of adsorbed film continuously increase upon time. The increase in Ed values for mixed systems with adsorption time should be associated with biopolymer adsorption at the interface [29]. Ed did tend to a plateau value at 10000s, with a similar behavior at long adsorption times, no matter the E4M bulk concentrations.
Mixed systems containing 1% of WPC and 1 × 10-2% of E4M showed a singular behavior, differences in the evolution of mixed films solid character was observed before 5000s (Figure 3b). WPC 1%+E4M 1 × 10-2% manifested a lag period for the adoption of a film with Ed values similar to the generated by pure WPC. In these mixed systems Ed is mainly determined by E4M, which forms less elastic films than single WPC films. After 5000 s, WPC adsorption increased, as protein dominates the Ed behavior of mixed films showing stronger solid character. Such a lag period was also evident in the Ed values corresponding to heated WPC 1%+E4M 1 × 10-2% mixed system, which could obey to aggregates formation in the solution bosom. WPC determined the final solid character (t>10.000 s) of mixed films since its bulk concentration was high enough to saturate the interface, including the monolayer collapse [30].
The polysaccharide influence was higher when E4M was able to saturate the air-water interface, i.e. WPC 1%+ E4M 1% (Figure 3c). In this case a lag period was also observed and the Ed values reached the corresponding to the single protein at long adsorption times. No difference was detected for heated WPC 1%+E4M 1% mixed system. In this case a slight decrease in Ed final values was observed, confirming the strong competence for the air-water interface as expected between these surface active biopolymers [30]. E4M dominated the adsorption process from the very beginning. The plateau in Ed evolution upon time occurred because the collapse of E4M film and the probable multilayer formation at short adsorption times preventing a further penetration of biopolymer molecules (WPC or E4M) [9,10].
Ed and tan δ will be described in detail in the next section; however, it can be observed a huge difference between the mixed systems as well as heating effects at long adsorption times.
Ed, which reflects the elastic character of formed films at long times, was determined by the protein content of mixed systems (Table 2). At the highest WPC concentration, higher Ed results were obtained. When heat was applied, as was seen above, an increase of these Ed values was observed. In fact, WPC determined the final solid character (t>10.000 s) of mixed films incrementing the liquid interface strength after heating.
In the other hand, relative viscoelasticity (tan δ) for adsorbed films could be attributed to the self-association of biopolymers molecules occurring at the air-water interface at long adsorption times. It was found that E4M higher concentration mixed systems, promotes a better association at liquid interface in this conditions, which enhances the heating process. However, when E4M could not saturate the air-liquid interface (WPC 1%+E4M 1 × 10-2%), a lower level of association between molecules would occur, leading to be destabilized by the heat treatment (Table 2).
Loss angle values variation for WPC/E4M films
Time-dependent loss angle for mixed systems are plotted in Figures 4a-c. The adsorbed films could be attributed to the self-association of biopolymers molecules occurring at the air-water interface [31]. Selfassociation process involves interaction between biopolymer hydrophobic groups, i.e. among protein-protein, E4M-E4M and WPC-E4M molecules. In the further case, interactions between methyl groups of E4M and the more hydrophobic residues of the protein would be occurring. When the polysaccharide bulk concentration was high enough to saturate the interface, 1% wt, loss angle values (Figures 4a and 4c) of unheated samples showed slight variation with time (Figures 4a and 4c). When the polysaccharide concentration was 1 × 10-2%, (Figure 4b) loss angle decreased with time. E4M due to its low bulk concentration exerted less influence on the WPC films. In fact, at this bulk concentration E4M adopted an expanded structure contributing to a reduction in Ev values [30].
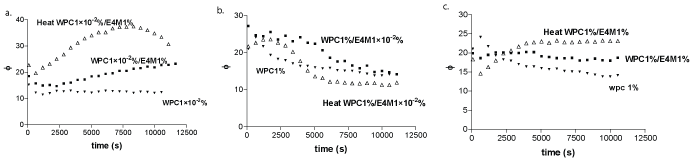
Figure 4: Loss angle (φ)) vs. time for alone WPC (close triangle), untreated (square) and heat treated (triangle) mixed systems of WPC 1 × 10-20/0%+E4M 1% (a), WPC 1%+E4M 1 × 10-20/0% (b), WPC 1%+E4M 1 % (c).
Remarkable differences were obtained for these samples after heat treatment. Thus, loss angle values decreased upon time for WPC 1% + E4M 1 × 10-2% wt, mixed systems (Figure 4 b). At the lowest protein bulk concentration (Figure 4a) the monolayer fluidification was observed from the starting of adsorption process. It has been reported that HPMC aggregates in aqueous solutions as the temperature is increased. The temperature at such process occurs was lower as the degree of methyl substitution increases. An increase content of hydroxypropyl groups increases the aggregation temperature [32]. The association is driven by hydrophobic interactions with a possible contribution from interchain hydrogen bond formation. Figure 4b illustrates the situation in which E4M could not saturate the air-water interface, WPC1%+E4M 1 × 10-2%. Loss angle resulted lower than the unheated samples. This process would occur in a lesser extent when the E4M was in the lowest concentration.
Foaming and interfacial relation of mixed systems
In the present section the possible relationship between foam properties and interfacial performance for mixed systems. Figures 5a-b shows the relation between OFC vs Kd and OFC vs Kp respectively. OFC vs Kd exhibits clearly the proportional increment of foamability with the diffusion (Kd ) for WPC 1%+E4M 1 × 10-2% heated sample system. A slight increment was detected for the other mixed systems.
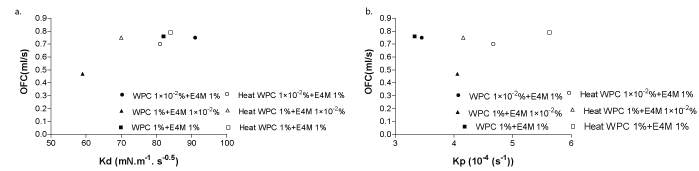
Figure 5: WPC 1 × 10-20/0% + E4M 1%; WPC 1%+E4M 1 × 10-20/0% and WPC 1%+E4M 1 % untreated systems (close symbols) and heat (H) treated systems (open symbols) for (a) Overall Foaming Capacity, OFC vs. Diffusion velocity, Kd correlation and (b) OFC vs Diffusion penetration velocity, Kp for same systems.
OFC vs Kp , shows an increase of OFC with the great increment of Kp for the previously mentioned mixed systems. No changes were registered for the remaining mixtures, after heat treatment for WPC 1%+E4M 1% as were seen in kinetics studies. OFC showed an increase just when E4M was present at the lowest solution concentration, i.e after heating mixed film formation would be favored as Kd and Kp were higher.
Figure 6 shows same relationship between t1/2 drain vs π180 min of adsorption time.
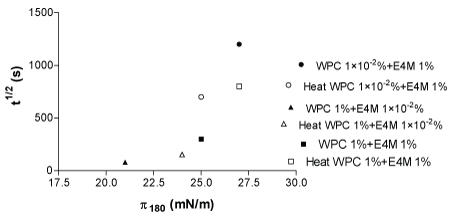
Figure 6: t1/2 drain vs π180 correlation for WPC 1 × 10-20/0%+E4M 1%; WPC 1%+E4M 1 × 10-20/0% and WPC 1%+E4M 1 % untreated systems (close symbols) and heat (H) treated systems (open symbols).
It can be observed a direct correlation with Figure 1e, because they are data from t1/2 drain, however, in this Figure 6 can be seen also the magnitude increase of π180 min for WPC 1%+E4M 1 × 10-20/0% and WPC 1%+E4M 1%, attributed to high protein content, as well as the surface pressure decrease for WPC 1 × 10-20/0%+E4M 1% after heat treatment.
Conclusions
In the present work foamability of WPC 1%+E4M 1 × 10-20/0 % heated mixed system showed the best performance and a very good correlation with diffusion velocity, first step for macromolecules adsorption at airliquid interface. On the other hand, same mixed system showed an increase of drainage stability that could be related with an extraordinary elastic character for film formation at long adsorption times.
WPC and E4M competed for the air-water interface as was demonstrated by the results obtained from dynamic measurements (π vs time evolution and rheology determinations). Although differences were observed according to the relative bulk concentration of biopolymers and the thermal treatment imparted (90ºC for 20 min). When the WPC could saturate the interface and E4M bulk concentration was low enough, E4M dominated the final equilibrium surface pressure. Heating increased π values at long adsorption time only at the higher protein concentration and the lower polysaccharide one. An additive or synergistic behavior was observed.
Considering the systems with the lowest WPC (1 × 10-20/0% wt) and E4M bulk concentrations of 1% wt, no remarkable effect could be observed. This finding would indicate that both biopolymers may coexist with the protein at the air-water interface contributing to surface pressure increase in a cooperative way. The solid character of mixed films did not show remarkable differences upon heating. In fact protein dominated the final Ed values when was present at 1%wt. The mixture effect decreases Ed at the lowest protein concentration.
In the presence of E4M, due to its outstanding surface activity, competitive adsorption would predominate when adsorbed in combination with WPC. The presence of the polysaccharide could also lead to concentration of adsorbed protein by a depletion mechanism because of the existence of a limited thermodynamic compatibility between both macromolecules in the vicinity of the air-water interface. There is an osmotic driving force that favors protein aggregation and that could result in a surface pressure increase [42]. Although one has to keep in mind that protein aggregation induced by heating increased the diffusion process, especially for WPC 1%+E4M 1 × 10-2, which was reflected in the overall foaming capacity. It is well known that thermal treatment can increase hydrophobicity of the aggregates structures formed.
These complex systems show realistic component relationships occurring in real foamed foods that support the need of getting a deeper knowledge in order to control their properties and their stability, which in turn will contribute to the design of new type of foods and textures.
Acknowledgments
This research was supported by the National Council of Scientific and Technical Research of the Argentine Republic (CONICET), University of Buenos Aires and Department of Chemical Engineering , Faculty of Chemistry, University of Seville.







