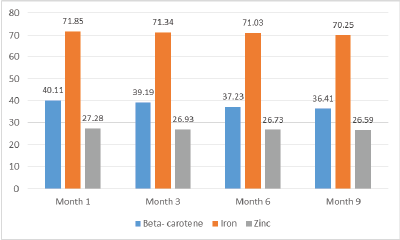
Figure 1: Change in micronutrient content (mg/100g) in dried amaranth leaves with storage (dry weight basis)


Peter Chege1* Judith Kimiywe2
1Department of Food, Nutrition and Dietetics, Kenyatta University, Kenya*Corresponding author: Peter Chege, Department of Food, Nutrition and Dietetics, Kenyatta University, Kenya, E-mail: chegepeterm@gmail.com
Vegetables are normally in plenty during the wet season with excess going to waste. However, there is scarcity during dry season. Vegetables can be preserved for consumption during the dry season. Solar drying is one of the vegetable preservation methods. Stored food can undergo nutrient loss and spoilage. Thus, this study aimed to assess the stability in terms of nutrient content and microbial load in dried amaranth leaves (Amaranthus cruentus) with storage in air tight containers for nine months at room temperature. Atomic Absorption Spectrophotometry was used for analysis of iron and zinc, whereas UV-VIS Spectrophotometry was used for β-carotene. Triplicate samples were analyzed for nutrient content and microbial load on a monthly basis. Differences in nutrient content in dried leaves after every three months up to nine months were established by use of t-test. Results show that the nutrient content for β-carotene, iron and zinc in fresh amaranth leaves was 5.75 ± 0.04, 8.47 ± 0.05 and 3.18 ± 0.04 mg/100g, respectively. These levels changed to 4.46 ± 0.04, 7.98 ± 0.02 and 3.03 ± 0.03, respectively after solar drying, but the change was not significant (P> 0.05). With nine months of storage, the concentration of β-carotene, iron and zinc remained relatively stable as shown by a small fluctuation which was not significant (P>0.05). E. coli and Msalmonella were absent. The levels for Coliforms, S. aureus moulds and yeasts were within the acceptable levels. Solar drying can be adopted as a vegetable preservation method due to minimal nutrient loss while microbial levels were within the acceptable levels. Thus, excess vegetables can be preserved to fill the seasonal gap.
Micronutrient; Microbial quality; Solar drying; Amaranth leaves
Kajiado County is one of the ASAL counties in Kenya, populated mainly by the Maasai pastoralists [1]. It experiences a bimodal form of rainfall, with short rains from October to December and long rains from March to May [2]. Crop production among Maasai pastoralists during the dry season is low [3]. Though amaranth grows naturally during the wet season it is also cultivated in some areas of the county.
To reduce these loses, there is need to dry and store leafy vegetables to enhance availability throughout the year and close the seasonality gaps [4]. Amaranth leaves are rich in micronutrients, but are highly perishable and have a short life span after harvesting [5]. During the wet season, amaranth leaves are abundant but without post-harvest preservation, the excess after consumption goes to waste [5]. Factors to consider on storage are to prevent loss of nutrients [6].
There is a need to explore appropriate ways that can be adopted by rural communities to preserve the leafy vegetables for use in dry seasons when availability is low. Solar drying has been documented as an appropriate method of drying vegetables [4]. Solar drying is recommended for preservation of green leafy vegetables over other methods as it is associated with minimal nutrient losses and leaves are protected against rain, dust and insects [7]. It is a cheaper and feasible method of preservation [8]. Though drying leads to loss of a proportion of the water soluble vitamins, fat soluble vitamins like β-carotene are fairly well-retained [5]. Solar drying has been found to have no significant effect on iron and zinc content in amaranth [9].
There are few literatures on the stability of dried amaranth leaves during storage, thus the need to determine the micronutrient and microbial quality assessment of solar dried amaranth leaves. Thus, this study aimed to assess the change in some nutrient content and microbial load in solar dried amaranth leaves after nine months of storage.
The amaranth cultivar used was Amaranthus cruentus due to its high yield and agronomic desirability. The leaves were obtained from Enkorika area in Kajiado County, Kenya which is an arid area.
The leaves were harvested from ten randomly selected farms at the 6th week after germination, in the morning, for optimal nutrients using the zigzag sampling method. The samples were packaged in perforated bags, placed in a cool box and transported to Kenya Industrial Research and Development Institute. The leaves were washed with cold clean running water followed by blanching.
The leaves were then spaced on the drying trays without overlapping and then placed into the solar tent dryers to dry for 12 hours until a moisture content of 6% was attained. Moisture content was determined using AOAC (934.01) method. Solar tent dryers were used as they provide a faster drying rate and keeping off insects. Milling was done using 0.65 mm mesh. The leaves were then packaged in packs of 50 g in sealed polythene bags to ensure no moisture absorption and transported to the laboratory for analysis.
Determination of nutrient content for β-carotene was analyzed by the use of UV-VIS Spectrophotometry (Model UV-1800). Crystalline-carotene type (IV) standard was obtained from Kobiac chemicals in Kenya. Other chemicals like potassium hydroxide, anhydrous sodium sulphate, sodium chloride, butylatedhydroxytoluene (BHT), dichloromethane, acetone, hexane, methanol and diethylether were sourced from local laboratories. To extract β- carotene, 50 ml of acetone–hexane mixture containing 0.1% BHT was added to 5 g sample and the mixture shaken for 10 minutes, centrifuged and decanted to a separating funnel. The supernatant was saponified by adding 25 ml of 0.5M methanolic potassium hydroxide. It was then shaken and allowed to settle for 30 minutes, then washed with 100 ml portions of distilled water while discarding the aqueous layer continuously. The extract was then dried by filtering over anhydrous sodium sulphate. The filtrate was concentrated in a rotary evaporator at 45°C and reconstituted in methanol to 50 ml. Different concentrations of standard solution were prepared using 95% UV β - carotene type 1 (Sigma Chemicals). A stock solution of (100 µg/ml) was made by dissolving 0.01 g of β - carotene standard into 10 ml hexane, which was then increased to 100 ml. The working standard solution was used to prepare standard solutions of various concentrations (1-12 µg/ml). The absorbance (A) of each concentration was measured using the HPLC at 450 nmג.
Iron and zinc were determined using Atomic Absorption Spectrophotometry (Shimadzu AA-680). One (1) gram of sample was weighed into a digestion tube. Concentrated nitric acid (5 ml) was added to the sample and heated. Hydrogen peroxide (30%) was added to the digestion mixture until it became clear. The clear solution was then made up to 50ml with a volumetric flask. A working solution of 10 ml of 1000 ppm (stock solution) was put into 100 ml flask and topped up to 100 ml mark with distilled water. A calibration standard for iron was prepared by adding 0, 2, 4, 6 and 8 ml of the working standard solution into100ml volumetric flask and topping up to 100 ml using distilled water. Both the samples and the standards were aspirated for analysis. A plot of calibration graph of concentration (ppm) against the absorbance was made. From the calibration curve the absorbencies of the samples were extrapolated to determine the concentration of iron in the samples. This procedure was repeated for zinc analysis.
The growth media (agar and broth) was prepared and sterilized in an auto clave (121°C for 15 minutes). The growth media was cooled to about 48°C in a water bath. Serial dilutions were conducted using sterile Phosphate Buffered Peptone Water (BPW) and the inoculum cultured into the respective media using pour plate and spread plate methods. The initial dilution was achieved by aseptically weighing flour samples (25 g) and 225 ml of the dilution media added. The homogenized sample (1 ml) for each dilution was then added into each of the appropriately marked duplicate petri dishes. To the pour plates, about 20 ml of the cooled media per 100 × 15 mm plate was poured to ensure a thickness of 0.3 cm swirl plates to mix. Spread plates were prepared by pipetting 0.1 ml of the inoculum on the solidified agar plates with a sterile glass rod. The inoculum was evenly distributed on the plate using a sterilized glass spreader. The spread plates were then inverted and incubated for 24 hours (37 °C) under aseptic conditions and the Colony Forming Units (CFU) counted using a colony counter for microbial load determination. Colonies were counted only on plates that had between 30 and 300 colonies. The number of colonies was multiplied by the number of times the original ml of bacteria was diluted divided by the volume of the culture plate. This is formulated as; CFU /ml = (no. of colonies × dilution factor)/volume of culture plate. To determine the microbial shelf-stability of the flour samples, the microbial load was determined after every 3 months for a period of nine months in triplicate.
Total viable counts were determined by pour plating on nutrient agar. One (1) ml of culture was pipetted into a sterile petri plate; melted agar medium was then added and mixed well by gently swirling the plate on the table top. Because the sample is mixed with the molten agar medium, a larger volume can be used than with the spread plate. The plate is inverted and cultured at 37 °C for 24 hours.
For the moulds and yeast counts, the samples were spread plated on Potato Dextrose Agar and incubated at room temperature (28 °C) for 5 days. Only plates with 10-150 colonies were counted.
Total coliform counts were determined by pour plating on MacConkey Agar plates and incubated at 37°C for 24 hours. Red colonies were counted using a colony counter. Total coliforms are grown in lactose medium, at a temperature of 37°C. They are provisionally identified by the production of acid and gas from the fermentation of lactose.
E- coli pre-enrichment was done by the addition of 1 ml of the food homogenate into 9 ml of nutrient broth at 37°C for 24 hours followed by selective enrichment using Tergitol -7 Agar at 37°C for 24 hours with positive colonies identified as golden yellow colonies. This was followed by streaking into EMB agar. Positive colonies showed a green metallic sheen.
Salmonella pre-enrichment was done using Buffered Peptone Water (25 g sample flour: 225 ml of BPW) and incubated at 37°C for 24 hours. Selective enrichment was done by taking 1 ml of the sample into 10 ml of Selenite Cysteine Broth and incubated at 37°C for 24 hours. This was followed by plating in Brilliant Green Agar at 37 °C for 24 hours to obtain isolated colonies. Suspect colonies were cubated atidentified using serological tests. A loop full of the culture was transferred into 5 ml of Brain Heart Infusion Broth and in 37 °C until visible growth occurred. Formalized physiological solution (2.5 ml) was added. Serological confirmation tests using polyvalent antisera for flagellar (H) and somatic (O) antigens were carried out. Samples showing agglutination of both H and O antisera were identified as positive for Salmonella spp.
About 50 ml Buffered Peptone Water by adding 10 gm of sample was inoculated and incubated at 37 °C for 18 hours. 10 ml of the incubated sample was transferred to 100 ml of Tetrathionate Broth and incubate at 35°C. After 24 and 48 hours, it was sub-cultured to Brilliant Green Agar incubate the plates for 18 hours at 37°C.
The enumeration of S. aureus was done by pipetting 1 ml of the food homogenate to the petridish and pour plating done using Baird Parker Agar. The samples were incubated for at 37°C for 24 hours. Positive colonies showed shiny black colonies with grey margin.
Data were analyzed in SPSS (version 20.0) software. Paired t-test for independent samples was used to determine if there was a significant difference between the nutrient content and microbial levels in the dried amaranth leaves in month 1 and month 9. Significance levels was determined at 95% confidence interval where a p-value of <0.05 was considered significant.
The study noted slight changes in nutrient content from month one to month 9 of storage (Figure 1).

Figure 1: Change in micronutrient content (mg/100g) in dried amaranth leaves with storage (dry weight basis)
Table 1 show the mean change in nutrient content was 3.7 ± 0.04 mg/100g, 1.4 ± 0.03 mg/100g and 0.69 ± 0.04 mg/100g for β-carotene, iron and zinc, respectively. These losses translated to a percentage loss of 9.1%, 2.0% and 2.8% for β-carotene, iron and zinc, respectively. From the results, there was a slight change in the nutrient content at the ninth month for β-carotene, iron and zinc which were not significant (P>0.05).
|
Mean ± SD |
|||
(n=3) |
Day 1 |
9th month |
Loss (%) |
t-test (P value) (month 0 and 9th month) |
β-carotene |
40.11 ± 3.21 |
36.41 ± 3.08 |
3.7 (9.2%) |
0.57 |
Iron |
71.85 ± 3.93 |
70.25 ± 3.26 |
1.4 (2.0%) |
0.65 |
Zinc |
27.28 ± 1.43 |
26.59 ± 1.22 |
0.69 (2.5%) |
0.62 |
Table 1: Micronutrient content (mg/100g) in dried amaranth leaves with storage (dry weight basis)
The microbe levels assessed were coliforms, Escherichia coli, Staphylococcus aureus, salmonella, yeasts and moulds (Table 2).
(n=3) |
Levels (cfu/g) |
Recommended levels (ICMSF) |
|||
Microbes |
At start |
Month 3 |
Month 6 |
Month 9 |
Limit (Max) |
Total plate count |
2.0x102 |
3.1x102 |
4.4x103 |
7.8x103 |
105 |
Total coliforms count |
3 |
2.1x101 |
4.9x101 |
5.2x101 |
102 |
E. coli |
Nil |
Nil |
Nil |
Nil |
Nil |
S. aureus |
5 |
3.4x101 |
1.2x102 |
2.3x102 |
105 |
Salmonella |
Nil |
Nil |
Nil |
Nil |
Nil |
Yeasts & Moulds |
1.2x101 |
3.7x101 |
5.8x101 |
7.4x101 |
104 |
Table 2: Microbiological Count of Solar dried amaranth leaves during storage
Total plate count increased from 2.0 × 102 to 7.8 × 103. The total coliforms increased from 3 to 5.2 × 101. The levels for S. aureus for 5 to 2.3 × 102. In addition, the levels for Yeasts & Moulds increased 1.2 × 101 to7.4 × 101. The levels of the other microbes by the end of nine months were within the levels recommended by the International Commission of Microbial Specification for Foods (ICMSF) [10]. The E. coli and salmonella were absent.
There was no significant change in the amount of beta-carotene with nine months storage. This is in agreement with a study by Prabhu and Barrett [11] that noted no change in beta-carotene content with storage of leafy vegetables. Similarly, there was no significant change in the amount of iron and zinc which agrees with a study by Negi and Roy [12] and Makobo, Shoko & Mtaita, [5] indicated no significant decline in these nutrients with storage. Thus, solar drying of amaranth leaves is an appropriate method for leafy vegetable preservation as it is associated with minimal loss of nutrients. Adequate amounts of ß- carotene, iron and zinc are retained.
There was a slight increase in the levels of total plate count, total coliforms count,S. aureus, Yeasts and Moulds. E. coli, Salmonella were absent. The levels by the end of nine months were within the levels recommended, suggesting shelf stability. Thus, dried amaranth leaves are safe for consumption when stored for nine months and thus can fill the seasonal gap. This finding is similar to other studies that established that storage of leafy vegetables up to one year does not pose any microbial threat [13,14]. Solar drying has therefore been shown to be capable of elongating the shelf-life of dried amaranth leaves and thus ensure the maintenance of safe, good quality during proper storage for at least nine months. Thus, solar drying is an effective method of food preservation as it prevents the growth and multiplication of micro-organisms.
Drying amaranth leaves and storing them in airtight containers at room temperature is sufficient to enhance stability for nine months without any significant change in the nutrient contents for β- carotene, iron and zinc. The stability of the micronutrients is a key factor in the success of food storage. Solar dying could thus be considered as an innovative way of keeping the overall quality of amaranth leaves and can ensure availability of amaranth leaves up to the next season.
The study recommends solar dried amaranth leaves to be stored in sealed containers at room temperature to ensure nutrient stability and microbial safety as a preservation method for closing seasonality gap.
Download Provisional PDF Here
Article Type: Research Article
Citation: Chege P, Kimiywe J (2016) Micronutrient and Microbial Quality Assessment of Solar Dried Amaranth (Amaranthus Cruentus) Leaves Produced in Kajiado County Kenya. Nutr Food Technol Open Access 3(1): doi http://dx.doi. org/10.16966/2470-6086.135
Copyright: © 2016 Chege P, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access