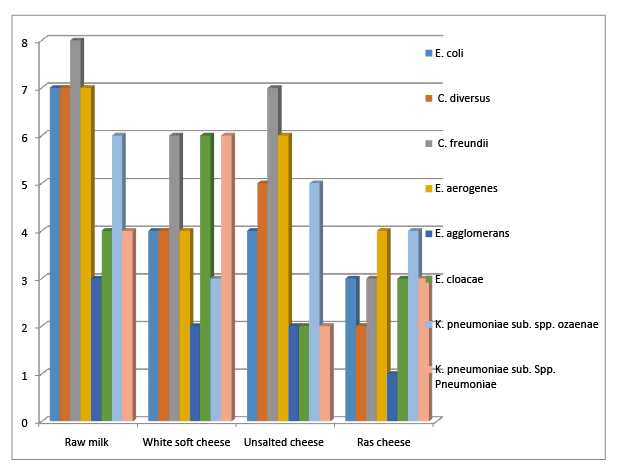
Figure 1: Incidence of coliform organisms in different cheese samples

Abd El Rahaman M Elbagory*1 Ahmed M Hammad1 Alzahraa2 MA Shiha2
1Department of Food Hygiene and Control, Faculty of Veterinary Medicine University of Sadat City, Egypt*Corresponding author: Abd El Rahaman M Elbagory, Department of Food Hygiene and Control, Faculty of Veterinary Medicine University of Sadat City, Egypt, E-mail: Elbagory200@yahoo.com
The present study investigated the prevalence of coliforms, antibiotic resistant coliforms and E. Coli serotypes in raw milk and some varieties of raw milk cheese in Egypt. One Hundred and twenty samples of raw milk and white soft cheese (salted and unsalted) and hard cheese (Ras) collected from different areas in El Minofia governorate, Egypt were examined. The obtained results declared that coliform members including E. Coli, Citrobacter diversus, C. freundii, Enterobacter aerogenes, E. agglomerans, E. cloacae, Klebsiella pneumoniae sub. spp. ozaenae and K. pneumoniae sub. spp. pneumoniae were isolated at varying percentage from examined samples. Kirby-Bauer disk diffusion test was used to examine bacterial susceptibility to ten antimicrobial agents including chloramphenicol, impenem, sulbactam+cefoperazone, ampicillin+sulbactam, amoxicillin+clavulanic acid, cefotaxime, gentamycin, amikacin, cefoperazone and tetracycline. Some strains showed multidrug resistant phenotype. The previously tested coliform strains, in order, showed resistance to AMC ; SAM & AMC ; AMC & CFP ; AMC & TE and C, AMC, CFP & TE antibiotics, while each showed sensitivity to the other tested antibiotics. The isolated E. Coli proved to belong to 5 serotypes; O111:K58 (B9 ) EHEC, O124:K72 (B17) EIEC, O119:K69 (B19) EPEC, O55:k59 (B5 ) EPEC and untypable strains. In conclusion, this study provides meaningful data on the incidence of coliforms in raw milk cheese in Egypt and merely underlines the need for implementation of strict hygiene measures in preparation of raw milk cheese in Egypt. The most alarming trend detected in this study was the determination of antibiotic resistant and potentially toxigenic E. Coli strains. This discovery makes ongoing surveillance of coliforms a high priority to identify emerging harmful strains within food production.
Coliforms; Antibiotic resistant coliforms; E. Coli serotypes; Cheese
Although milk has an outstanding nutritional quality, yet it is also an efficient vehicle for transmission of food borne pathogens that pose a serious threat to human health, and constitute about 90% of all dairy related diseases [1]. Coliforms particularly E. Coli are frequently used in the microbiological analysis of food as an indicator of poor hygienic condition. Moreover, coliform test is used to measure the quality of practices used to minimize microbial contamination of dairy products and as an approved safety indicator in HACCP system [2,3]. Contamination of cheese with coliforms especially fecal coliforms gives an indication of either direct or indirect fecal contamination and considered as a mirror for the degree of disregard of numerous hygienic rules during processing and marketing [4]. Some members of coliforms are responsible for the development of objectionable taints in milk and its products rendering them of inferior quality or even unmarketable, moreover certain serotypes of E. Coli are associated with gastroenteritis and food poisoning outbreaks [2].
Antibiotic resistant coliforms have increased worldwide that leading to failures in treatment of human infectious diseases and they are a major concern in the anti-infective therapy of both humans and animals. Moreover they are considered dangerous healthy, social and economic problem in the world where antibiotic resistance of coliforms have a biological danger, which increases the disease in animals and human [5]. Therefore the present study is planned to isolate and identify coliforms, serotyping of isolated E. Coli and determination of antibiotic resistance for various members of isolated coliforms.
One hundred and twenty samples; 30 each of raw milk, white salted soft, white unsalted soft cheese and Ras cheese were collected from dairy shops from different places in Menofia province, Egypt in clean sterile polyethylene bags and transferred in ice box to the laboratory with a minimum of delay to be examined. Each cheese sample was thoroughly mashed, then 25 grams from each prepared sample was aseptically added into 225 ml of 2% Sodium citrate and thoroughly mixed till completely emulsified.
The technique recorded by APHA [6] was used for isolation of coliforms from prepared raw milk and prepared cheese samples. Isolated purified colonies of coliforms were identified morphologically and biochemically according to Holt et al. [7].
Isolated strains of E.coli were identified serologically, using polyvalent and corresponding monovalent antisera manufactured by Denka Seiken Ltd. Japan for Oxoid Ltd. where two separate drops of sterile distilled water were put on a clean glass slide and a portion of the colony from the suspected culture was emulsified with sterile loop in each drop of sterile distilled water on the slide to give a smooth fairly dense suspension. After that, to one of the two suspensions, one drop of sterile distilled water was added and mixed to be used as negative control while one drop of polyvalent antiserum was added to the other one and titled backward and forward up to one minute. Agglutination was observed when a strongly positive agglutination with one of pools of polyvalent serum. A further portion of the colony was inoculated into nutrient agar slant and incubated for further growth to get a culture for testing with monovalent sera. A heavy suspension of organisms from each slope culture was prepared in distilled water and slide agglutination test was performed with O and K sera to identify the O and K antigens. E. Coli polyvalent 2, 3 and 4 and E. coli monovalent antisera; O26: K60 (B6), O44: K74 (B-), O55: K59 (B5), O78: K80 (B) O86: K (B7), O11: K58 (B9), O112: K66 (B1), O114: K9, O119: K69 (B19), O124: K72 (B17), O125: K70 (B15), O126: K71 (B16), O127: K63 (B8) O128: K67 (B12), O142: K86and O157:H7 were used.
Characterized strains were tested for their susceptibility to 10 antimicrobial agents by the disc diffusion method according to the guidelines of the Clinical and Laboratory Standards Institute [8]. The antimicrobial agents tested were chloramphenicol (30 µg), impenem (10 µg), sulbactam+cefoperazone (105) µg, ampicillin+sulbactam (20 µg), amoxicillin+clavulanic acid (30 µg), cefotaxime (30 µg), gentamycin (10 µg), amikacin (30 µg), cefoperazone (75 µg) and tetracycline (30 µg). The zone diameter for individual antimicrobial agents was then translated into susceptible, intermediate and resistant categories according to CLSI [8].
Data in figure 1 showed that the incidence of the isolated coliforms in the examined raw milk samples were E. Coli (7/30, 23.33%), Citrobacter diversus (7/30,23.33%), C. freundii (8/30, 26.67%), Enterobacter aerogenes (7/30, 23.33%), E. agglomerans (3/30, 10 %), E. cloacae (4/30, 13.35%), Klebsiella pneumoniae sub. spp. ozaenae (6/30, 20%) and K. pneumonia sub spp. pneumoniae (4/30 13.33%). While the isolated coliforms in the examined salted white soft cheese samples were E. Coli (4/30, 13.3%), Citrobacter diversus (4/30, 13.3%), C. freundii (6/30, 20%), Enterobacter aerogenes (4/30, 13.33%), E. agglomerans (2/30, 6.67%), E. cloacae (6/30, 20%), Klebsiella pneumoniae sub. spp. ozaenae (3/30, 10%) and K. pneumonia sub spp. pneumoniae (6/30 20%). Coliforms other than E. Coli could be isolated with different percentage by Digrak & Ozcelik and Amer et al. [9,10]. The incidence of E. Coli substantiated what have been recorded by Nichols et al. and El-Gamal & Abdel-Khalek [11,12]. It has been demonstrated that K. pnumoniae is an important cause of nosocomial infections like pneumonia, septicaemia, urinary tract and soft tissue infections [13,14]. K. oxytoca was reported as enterotoxigenic microorganism and cause an antibiotic associated hemorrhagic colitis [15]. Some species of genus Enterobacter as E. zakazakii, unlike other enteric organisms, may cause highly lethal syndrome of bacteremia and meningitis with central nervous involvement in neonates [16]. Moreover the horizontal transfer of Shiga-like toxin genes to some species of genus Citrobacter as C. freundii which was responsible for an outbreak among school children in Germany, further highlight the public health importance of coliforms [17]. Data in figure 1 showed that the incidence of the isolated coliforms in the examined unsalted soft cheese were E. Coli (4/30, 13.3%), C. diversus (5/30, 16.67%), C. freundii (7/30, 23.33%), E. aerogenes (6/30, 20%), E. agglomerans (2/30, 6.67%), E. cloacae, (2/30, 6.67%), K. pneumonia sub. spp. ozaenae (5/30, 16.67%) and K. pneumonia sub. spp. pneumoniae (3/30, 10%). Coliforms other than E. coli could be recorded by Moustafa , Zeinhom and Ahmed [18,19,20]. The incidence of E. Coli in examined samples agree to some extant to what have been recorded by Hamid & El Owni [21]. The high rate of contamination in this type of cheese may be due to handling, transportation or storage in a place contaminated with these types of bacteria or during processing of cheese. This type contain no salt, where salting process is important in preservation and as a flavor enhancer, reducing the moisture content in the cheese [22] and suppress the growth of undesirable microorganisms [23].

Figure 1: Incidence of coliform organisms in different cheese samples
Data in figure 1 showed that the incidence of the isolated coliforms in the examined hard cheese (Ras or Roomy cheese) were E.coli (3/30, 10%), Citrobacter diversus (2/30, 6.67%), C. freundii (3/30, 10%), Enterobacter aerogenes (4/30, 13.34%), E. agglomerans (1/30, 3.33%), E. cloacae (3/30, 10%), Klebsiella pneumonia sub. spp. ozaenae (4/30, 13.33%) and K. pneumoniae sub. spp. pneumoniae (2/30, 6.67%). Similar incidence of E. coli in examined samples was recorded by Elessawy [24]. Similar coliforms other than E. Coli could be recorded by Mohamed , Hassan and Sadek et al. [25-27]. The stages of processing of Ras cheese may minimize the risk of contamination and result in low level of contamination, but may be post processing as during transportation, storage or handling by unclean handlers [28].
The results obtained in figures (2-6) explained the antibiotic resistance to coliform isolates including E. Coli that isolated from milk and cheese samples. The tested coliform strains, in order C. diversus, C. freundii, E. cloacae, K. pneumoniae sub. spp. pneumoniae and E. Coli O111 showed resistance to AMC; SAM & AMC; AMC & CFP; AMC&TE and C, AMC, CFP&TE antibiotics, while each showed sensitivity and intermediate resistance to the other tested antibiotics. Wang et al. [29] pointed out that the most examined C. freundii isolates were resistant to gentamicin, tobramycin, and aztreonam. The results indicated that the susceptible rates of C. freundii to aminoglycosides and ciprofloxacin are decreased markedly. Yagoub et al.[30] declared that the examined Enterobacter spp. isolated from raw milk samples had a wide range of resistance to penicillin, clindamycin, amoxicillin and ampicillin, while Farzana et al. [31] showed that the tested Enterobacter isolate was sensitive to nalidixic acid, tetracycline and showed little resistance to chloramphenicol. Klebsiella spp. isolate was resistant to nalidixic acid and little to chloramphenicol as have been showed by Farzana et al. [31], while Chauhan et al. [32] illustrated that the tested Klebsiella spp. isolates showed susceptibility to imipenem followed by ciprofloxacin, piperacillin/tazobactam combination and ciftazidime. The imipenem antibiotic to which Klebsiella spp. was highly sensitive and is a drug of choice. Nam et al. [33] recorded that C. freundii, E. cloacae and Klebsiella pneumoniae isolates have strong antimicrobial activity against amikacin, gentamicin, and piperacillin whereas rifampin, cephalothin, cefazolin, and ampicillin were ineffective against most of the tested bacterial species. Paneto et al. [34] surveyed the occurrence of toxigenic E. coli isolated from raw milk cheese samples and tested its susceptibility to certain antimicrobial agents and found that the most frequent resistance was observed to the cephalothin, nalidixic acid, doxycycline, tetracycline and ampicillin, but all tested E. Coli isolates from raw milk samples exhibited 100% resistance to rifampin and tetracycline and 50% resistance to nalidixic acid but were100% sensitive against imipenem as recorded by Aftab Uddin et al. [35]. Sallam [36] recorded that E. Coli isolates were tested for susceptibility to eleven antibiotics; ampicillin, amoxicillin, amoxicllin/ clavulanic acid, oxytetracycline, tetracycline, doxycycline, ciprofloxacin, enrofloxacin, neomycin, gentamicin and erythromycin. The resistance to such antibiotics were 50%, 50%, 7%, 67%, 67%, 40%, 23%, 23%, 23%, 13%, and 100%, respectively. It is evident from the last few years that some strains of Klebsiella, Enterobacter and Citrobacter spp. had been isolated from stools and the intestinal contents of both children and adults in several epidemiological studies of acute and chronic disturbances. Moreover, certain members of Citrobacter spp. had been suspected to cause enteric infection. C. freundii had been found among urinary and other pyogenic infections in humans [37]. Also, there is a loose association between E. coli and possible enteric pathogens [10]. Klebsiella pneumoniae occurs as a commensal in the upper respiratory tract and in the intestine and may be associated with catarrhal conditions in the respiratory tract, paranasal sinusitis, conjunctivitis and pneumonia in man [38].
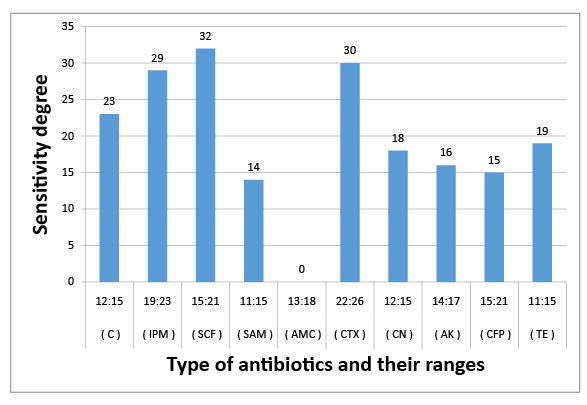
Figure 2: Susceptibility of C. diversus to certain types of antibiotics
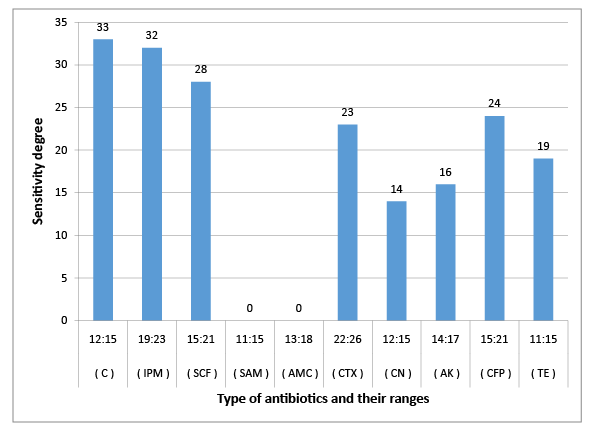
Figure 3: Susceptibility of C. freundii to certain types of antibiotic
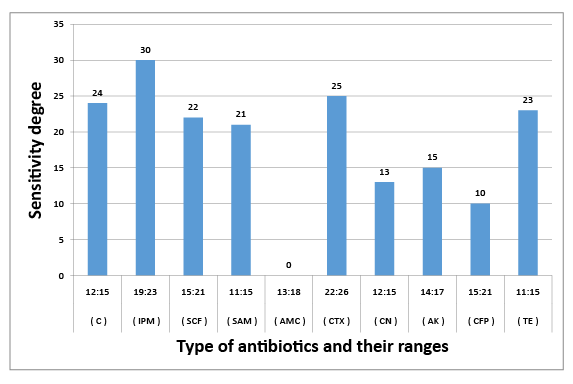
Figure 4: Susceptibility of E. cloacae to certain types of antibiotic
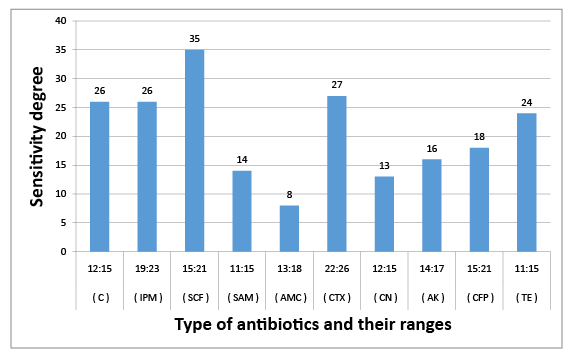
Figure 5: Susceptibility of K. pneumoniae sub. spp. pneumoniae to certain types of antibiotics
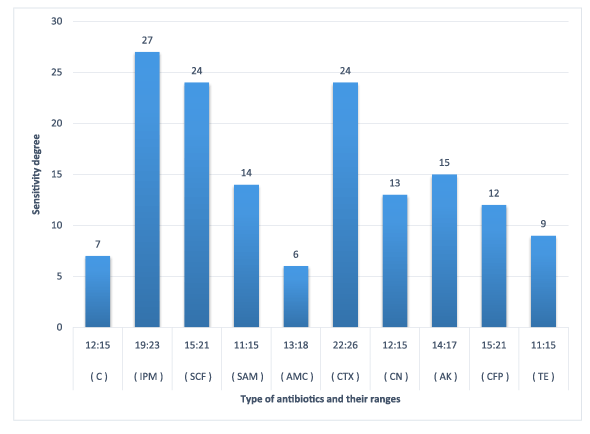
Figure 6: Susceptibility of E. Coli O111:K58 to certain types of antibiotics
It’s evident from the results recorded in table 1 that some isolated E. Coli strains could be serologically typed into serotypes O111 (3 strains), O119 (2 strains), O55 (one strain) and O124 (one strain). These findings substantiated what have been found by Saudi and Moawad, Ahmed and Sallam and ElShinawy et al.[39-41]. E. Coli has been established to be among the etiological agents causing enteritis and several extra gastrointestinal diseases. Also, the organisms are recognized as pathogens for human and ruminants [42]. In recent years, much attention has been paid towards E. Coli because of its importance as an organism of true fecal origin with the possible existence of associated enteric pathogens. Also, the public health hazard of enteropathogenic E. Coli has been emphasized by many investigators as they have been implicated in human cases of gastroenteritis, epidemic diarrhea in infants, sporadic diarrhea in children as well as in cases of food poisoning [43]. However 6 main types of diarrheagenic E. Coli have been associated with food borne disease; enterotoxigenic E. Coli (ETEC) which is responsible for watery diarrhea (travelers’ diarrhea) as consequence to production of heat labile toxin (Cholera like toxin) and heat stable toxin (diarrheal toxin). Enteropathogenic E. Coli (EPEC) which is associated with infantile diarrhea, Enterohaemorrhagic E. Coli which may result in bloody diarrhoea, hemorrhagic colitis, hemolytic uremic syndrome and thrombocytopenic purpura. Enteroaggregative E. Coli (EaggEC) which aligns themselves in parallel rows on either tissue cells or glass described as ‘stacked brick-like’ then elaborate heat labile toxin, antigenically related to haemolysin and a plasmid encoded that stables toxin resulting in persistent watery diarrhea especially in children. Enteroinvasive E. Coli (EIEC) which may result in fever and profuse watery diarrhoea containing mucous and streaks of blood. Diffusely adherent E. Coli (DAEC), have been associated with diarrhea in some studies but not consistently [44].
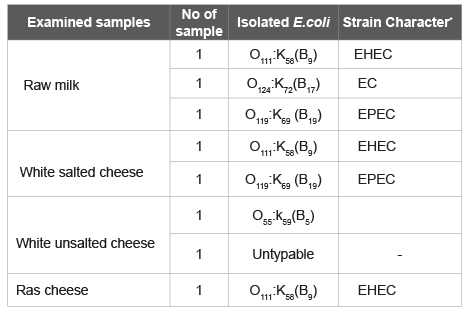
Table 1: Serological identification of isolated E. Coli of examined samples.
In conclusion, this study provides meaningful data on the incidence of coliforms in raw milk cheese in Egypt and merely underlines the need for implementation of strict hygiene measures in preparation of raw milk cheese in Egypt. The most alarming trend detected in this study was the determination of antibiotic resistant and potentially toxigenic E. Coli strains. This discovery makes ongoing surveillance of coliforms a high priority to identify emerging harmful strains within food production.
Download Provisional PDF Here
Article Type: Research Article
Citation: Elbagory AM, Hammad AM, Alzahraa, Shiha MA (2016) Prevalence of Coliforms, Antibiotic Resistant Coliforms and E. Coli Serotypes in Raw Milk and Some Varieties of Raw Milk Cheese in Egypt. Nutr Food Technol 2(1): doi http://dx.doi. org/10.16966/2470-6086.114
Copyright: © 2016 Elbagory AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access