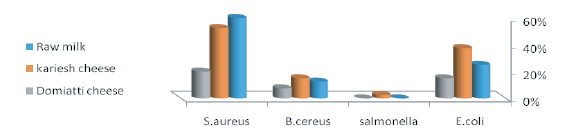
Figure 1: Incidence of E. coli, Salmonella, B.cereus and S. aureus in examined raw milk and soft cheese (Kareish and Damietta cheese) samples.


Elbagory AM1,* Sh. Elshazly Eman2 K Fathalla Eman2
1Department of Food Hygiene & Control, Faculty of Veterinary Medicine, University of Sadat City, Egypt*Corresponding author: Elbagory AM, Department of Food Hygiene & Control, Faculty of Vet. Med, University of Sadat City, Egypt, E-mail: elbagory200@yahoo.com
One hundred and twenty samples, 40 each of raw milk, Kareish cheese (raw skim milk soft cheese) and Damietta cheese (white raw milk soft cheese) were collected from supermarkets, dairy shops and street vendors in El- Gharbia governorate, Egypt for microbiological investigation.
The obtained results revealed that S. aureus, E. coli and B. cereus could be detected in 60, 52.5 & 20%; 25, 37.5 & 15% and 12.5, 15 & 7.5% of examined samples, respectively. Serological typing of fourteen strains of isolated E. coli proved to belong to ten serotypes; O159, O142, O18, O15, O125, O111, O55, O119, O26 and O128. Salmonella typhimurium could be isolated from one Kareish cheese sample (2.5%) with serological typing proved that the strain has antigenic structure of O: 1, 4, 5, 12, H: i: 1, 2. Concerning the antimicrobial activities of some probiotic bacteria; Lactobacillus planterum (p1 ), Lactobacillus acidophilus (p7 ), Lactobacillus brevii (p102) and Lactobacillus casii (l.c) using an agar well diffusion assay against selected isolates of E. coli, B. cereus, S. aureus and Salmonella typhimurium indicated that the used probiotic bacteria appeared to have antimicrobial effect against E. coli and B. cereus, while induce no antimicrobial effect against S. aureus and Salmonella typhimurium. The same probiotic strains were injected with E.coli in sterilized skim milk as in vitro experiment and the results showed a significant reduction in the count of E. coli during the periods of incubation.
S. aureus; E. coli; B. cereus; Raw milk; Soft cheese
Milk and its products including cheese are preferred as an excellent food for human of all ages as they contain all the nutrients required for rapid growth and maintenances of body health [1]. On the other side, milk and its products have been shown to be an ideal media for growth and multiplication of many microorganisms as E. coli, S. aureus, salmonella species and B. cereus that may constitute public health hazards for consumers [2]. S.aureus is still an important cause of food borne intoxications worldwide and it’s infection is estimated to be present in up to 90% of dairy farms and is responsible for 35% of the economic loss in the dairy industry, that constitute a serious problem in dairy industry [2,3]. E. coli is among large number of microorganisms which can access to milk and some dairy products and considered a reliable indicator of contamination by manure, soil and fecal polluted water. It is of public health concern due to the possible presence of enteropathogenic and/or toxigenic strains which lead to severe gastrointestinal disturbance. Moreover, their presence in milk or its products point out to possible presence of other enteric pathogens as salmonella [4]. Salmonella are the major pathogenic bacteria in humans and animals and their species are leading causes of acute gastroenteritis in several countries [5]. The fecal wastes from infected animals and humans are important sources of contamination of the environment and the food chain [6]. B. cereus is widespread in nature and proliferates in different habitats as soil and growing plants, but it is also well adapted for growth in the intestinal tract of insects, mammals and in food such as raw cow milk and soft cheese [7,8]. B. cereus produces several virulence factors including toxins and enzymes as hemolysin, protease and lecithinase which are considered the most important factors [9]. Nowadays the use of probiotics should be a main target to hinder or inhibit the spread of food borne pathogens and to raise the immune response of the animals. This inhibition could be due to the production of inhibitory compounds such as bacteriocins or reuterin, hydrogen peroxide, the alteration of pH values by the production of organic acids and competitive adhesion to the epithelium [10]. Lacto bacilli are important probiotics recognized for their fermentative ability as well as their health and nutritional benefits fermentations [11].
One hundred and twenty samples, 40 each, of raw milk, Kareish cheese and Damietta cheese were collected from dairy shops and street vendors at El-Gharbia governorate, Egypt. Each raw milk sample was subjected to Storch’s test [12] to exclude samples proved to be heat treated and to yoghurt culture test “YCT” [13] to exclude samples proved to contain inhibitory substances. Each sample of Kareish and Damietta cheese was thoroughly mashed in a sterile electric mixer before being examined.
25 ml or g. from each collected and prepared sample were added to sterilized flask containing 225 ml buffered peptone water (BPW) and incubated aerobically at 37°C for 24 hrs. One ml from each incubated BPW tube was transferred to 5 ml MacConkey broth tube and incubated at 37°C for 24 hr. A loopful from each incubated broth tube was streaked on Eosin Methylene Blue (EMB) agar and incubated at 37°C for 24 hrs. Morphologically typical colonies were taken into nutrient agar slope and incubated at 37°C for 24 hrs before being identified according to MacFaddin [14]. Serological typing of isolated E.coli was done using the slide agglutination technique adopted by Edwards and Ewing [15] using available polyvalent and monovalent antisera.
25 ml or g. of each prepared sample were added aseptically to 225 ml of sterile buffered peptone water (pH 7 ± 0.1) before being incubated at 37°C for 18 hrs, as pre- enrichment step. One ml and 0.1 ml quantities from each previously pre- enriched sample were transferred to 10 ml of each selenite -f- broth tubes and Rappaport Vassiliadis broth tubes before being incubated at 37°C and 42°C for 24 hrs, respectively as enrichment step. Salmonella Shigella agar (Oxoid), Xylose Lysine Deoxycholate agar (XLD) plates were used as selective media where a loopful from each incubated enrichment tubes was streaked on triplicate plates of plating media before being incubated at 37°C for 24 h. Two typical colonies and one a typical colony were picked from each selective agar plate and transferred to slope agar and incubated at 37°C for 24 hrs before being identified morphologically and biochemically according to Baily and Scott [17]. Isolated salmonella were typed serologically according to Kauffman Whites Scheme [18] using the available antisera (Welcome Diagnosis, A division of the Welcome Foundation limited, Dariford, England, DA 15 AH).
One ml of milk or 1 g. of each prepared cheese sample was transferred to 9 ml of peptone water tubes (0.1%, PH 7.0). Loopfuls from each incubated tubes were streaked on Polymyxin-Pyruvate-Egg yolk-MannitolBromothymol blue Agar (PEMBA) and Mannitol Egg Yolk Polymyxin (MYP) plates. B. cereus appears as peacock blue colored colonies and pink colonies respectively surrounded by a zone of precipitation of egg yolk after incubation at 37°C for 24 hrs. Suspected colonies were purified before being morphologically and biochemically identified as recommended by Shinagawa [20].
One ml of milk or 1 g. of each prepared cheese sample was transferred to 9 ml of peptone water tubes (0.1%, PH 7.0). Loopfuls from each incubated tubes were streaked over a dry surfaces of Baired Parker [22] agar plates before being incubated at 37°C for 48 hrs. Suspected colonies of S. aureus colonies were picked up onto nutrient agar slants and incubated at 37°C for 24 hrs before being subjected to morphological and biochemical identification.
Cell free culture supernatant (CFS) of some probiotics was prepared by inoculating 20 ml MRS broth tubes supplemented with 0.05% cysteine (De Man, Rogosa and Sharp obtained from Lab M) with one ml of each of Lactobacillus plantarum (p1 ), Lactobacillus acidophilus (p7 ), Lactobacillus brevii (p102) and Lactobacillus casii (l.c) before being incubated overnight at 37°C under anaerobic condition. Another one ml of the previously prepared culture was sub-cultured again overnight in 20 ml MRS broth. Cells were removed by centrifuging at 14,000 rpm for 5 min (Sorvall RC6 PLUS, Thermo-electron Corporation, Asheville, NC, USA). The supernatant was sterilized by using 0.45 μm–pore size Seitz filter with single sheet to eliminate the possible presence of Antimicrobial activity of the obtained CFS of the Lactobacillus strains against some isolated pathogens; S. aureus, Salm. typhimurium, B. cereus and E.coli (O148, O18 and O159) were determined by agar well diffusion method. Under aerobic condition, agar plates were inoculated with 100 μl of each target bacteria after growing them in a broth and diluting appropriately. Wells (5 mm) were cut into the plated sand and 10 µl of cell free culture supernatant (CFS) was placed into each well. Plates were kept at cool temperature for 1 hr and then incubated at 37°C for 24 hrs. The antimicrobial activity was determined by measuring the diameter of the inhibition zone around the wells.
Lactobacillus strains were studied for their effect on the viability of E. coli (strain O159 was used). Sterilized skim milk was inoculated with 1% of lactobacillus strains (infective dose 70 × 106 cfu/ml) and 1% of E. coli (infective dose 55 × 106 cfu/ ml) as recorded by McFarland’s standards [26]. The cultures were incubated at 37°C for 0, 2, 4, 6, 8 and 24hrs. E. coli was enumerated on its selective media. The experiment was repeated 3 times, the mean and SE was calculated by T test (SPSS). PH values of skim milk were recorded by pH - meter GLP 21+ (CRISON).
Recovery of E. coli from raw milk is not only regarded as an indicator of fecal contamination but more likely as an evidence of poor hygiene and sanitary practices during milking and further handling. The presence of E. coli itself in milk and milk products as a possible cause of food borne disease is insignificant because E. coli is normally a ubiquitous organism. However, the occurrence of pathogenic strains of E. coli in milk products could be hazardous for consumers [27]. It is evident from Figure 1 that E. coli could be isolated from examined raw milk, Kareish cheese and Damietta cheese samples in incidence rates of 25%, 37.5% and15% respectively. Nearly similar findings were reported by Olfa et al. [28], Mayda and Fatma [1], Hosny et al. [29] and El Sayed et al. [30]. Higher incidence was reported by Bruce et al. [31], Nagah et al. [32], Bahareem et al. [33] and Ozen et al. [34]. E.coli is a good indicator of fecal pollution as it exists in the normal microflora of the intestinal tract of humans and warm blooded animals [28].

Figure 1: Incidence of E. coli, Salmonella, B.cereus and S. aureus in examined raw milk and soft cheese (Kareish and Damietta cheese) samples.
It is evident from the results recorded in table 1 that some of isolated E. coli strains (14 strains) could be serologically typed into ETEC include O159 (2 strains), O125 (one strain), O15 (2 strains) and O128 (one strain), EPEC include O142 (2 strains), O55 (one strain) and O119 (one strain) an EHEC include O18 (1 strain), O11 (2 strains) and O26 (one strain). Nearly similar results were obtained by Najand and Ghanbarpour [35] and Abike et al. [36]. E. coli strains can cause considerable losses of neonatal animals where Enterotoxigenic strains attach to the brush border membrane of the jejunum and ileum and cause watery yellow, white to grey diarrhea in neonatal animals. Enteropathogenic strains of E. coli form lesions that destroy the brush border of the small intestine and form pedestal structures so that the bacteria remain in contact with the cells and cause chronic, mucoid diarrhea. Enterohemorrhagic E. coli colonizes the colon and causes necrosis of the villi; diarrhea is mucoid, sometimes hemorrhagic, and seldom fatal but often recurrent even with treatment, resulting in dehydration and reduced growth. Necrotoxigenic strains produce a toxin called cytotoxic necrotizing factor (CNF) and although not much is known about the pathogenesis [37]. According to the Egyptian standard [38] which stipulated that milk and milk products must be free from E. coli, 10 (25%), 15 (37.5%) and 6 (15%) raw milk, Kareish cheese and Damietta cheese samples, proved to exceed this limit.
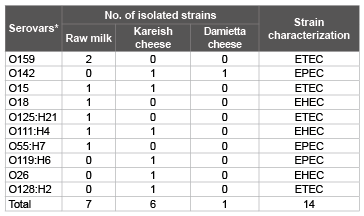
Table 1: Serotyping of some E. colis train from examined raw milk, Kareish
and Damietta cheese samples.
*Serotyping of the isolated strain of Salmonella showed S. typhimurium with
antigenic formula O antigen; 1, 4,5,12 and H antigen; i: 1, 2
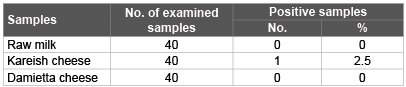
Table 2: Incidence of Salmonella isolated from examined raw milk and soft cheese (Kareish and Damietta cheese) samples.
Salmonella is one of the most important food-borne pathogens that can be transmitted through the consumption of contaminated milk and milk products. Early detection of salmonella in food is important for food safety [39]. Salmonella species are leading causes of acute gastroenteritis in several countries and salmonellosis remains an important public health problem worldwide, particularly in the developing countries. The situation is more aggravated by the ever increasing rate of antimicrobial resistance strains [40]. The result recorded in tables 2 and figure 1 showed that Salmonella organisms not detected in all the examined samples of raw milk and Damietta cheese. Similar findings were reported by Mhone et al. [41], Nero et al. [42] and Hamid and Owni [43], while Salmonella typhimurium which have antigenic formula of O,1,4,5,12 and H: 1,1,2 could be isolated from one (2.5%) of the examined Kareish cheese samples. According to Egyptian standard [38], Milk and milk products must be free from salmonella. Disinfection of cow teats, isolation of infected animals and effective cleaning of the milking equipment and utensils will reduce the potential of salmonella to enter the milk supply [44].
B.cereus causes problems to the foodstuff industry both by deteriorating the products and by endangering people’s health upon consuming contaminated foods [45]. The danger posed by some strains of this microorganism is increased by its ability to adapt to chemical [46], heat [47] and cold environment, as well as by toxin production.
Results in Figure 1 showed that B. cereus could be isolated from examined raw milk, Kareish cheese and Damietta cheese samples with incidence rate of 12.5%, 15% and 7.5% respectively. Nearly Similar finding was reported by Nemeckova et al. [48], El Sayed et al. [30]. Higher incidence rates were reported by Bassa et al. [49] and Hassan et al. [25], Eman et al. [50] and Zeinab et al. [51] as well as Citak et al. (2010) [52], while lower incidence rates were reported by Jackson et al. [53] and Hosny et al. [29].
Two kinds of food poisoning have been attributed to B. cereus; Emetic poisoning which is characterized by vomiting followed with diarrhea after 8 to 16 hrs in approximately a third of the cases, foods. The emetic toxin (cereulide) is produced in the food and poisoning occurs after ingestion of the toxin [54]. Diarrheal poisoning is characterized by watery diarrhea associated with abdominal pain and occurs within 8 to 16 hours after ingestion of the contaminated food. It is generally recognized that diarrheal poisoning occurred through production of enterotoxins in the intestine by ingested bacterial cells [55].
It is evident from figure 1 that S. aureus could be isolated from examined raw milk, Kareish cheese and Damietta cheese samples with incidence rates of 60%, 52.5%, 20% respectively. Nearly similar incidence rates were reported by Myada and Fatma [1] and Eman et al. [2] and El Sayed et al. [30]. Higher incidence rates were reported by Freitas et al. [56], Tondo et al. [57] and Ozen et al. [34], while lower incidence was obtained by Ekici et al. [58], El Sayed et al. [30] and El-baradei et al. [59]. S. aureus may gain access to milk from cows of mastitis, milk handlers and due to lack of hygiene during production. A high rate of growth can be reached quickly when milk is stored in the farm under unfavorable condition [60]. Its presence in food may constitute public health hazard as it may produce various types of enterotoxins (20 types of staphylococcal enterotoxins denoted A - U are currently known [61], in food which may result in food intoxication with sever vomiting and diarrhoea after short period (1–8 hrs) of ingestion of such food [62].
Over the past decade, there has been considerable progress in identifying potentially beneficial roles for probiotics in human health. Probiotics are defined as “live microorganisms administered in adequate amounts that confer a health effect on the host, these microbes are valuable to the host by improving the beneficial microbial population, which is, otherwise diminished by the use of antibiotics and also known as friendly bacteria or good bacteria. Probiotics are similar to the natural bacteria that our digestive system produce and travel through the digestive tract to our intestine [63].
Recorded data in table 3 showed that probiotic strains Lactobacillus planterum (p1 ), Lactobacillus acidophilus (p7 ), Lactobacillus brevii (p102) and Lactobacillus casii (l.c) were effective to inhibit the growth of B. cereus by inhibition zones (Agar well diffusion method) of 12, 10, 11 and 13 mm, respectively. Nearly similar results were obtained by Marie et al. [64]. They also had inhibitory effect against E. coli O159 by inhibition zones (9, 9, 8 and 4 mm), E.coli O142 (8, 8, 7 and 9 mm) and E. coli O18 (8, 6, 7 and 5 mm), respectively. Nearly similar results were obtained by Mami et al. [65] and Fahad and Ahmed [66], while they proved to be not effective against S. aureus and Salmonella typhymurium. Similar results were recorded by Mohan kumar [67]. The ability of lactic acid bacteria to inhibit the growth of pathogenic bacteria is well known. Lactobacilli are able to compete with pathogenic bacteria when they were incubated together [19], but the degree of inhibition was bacterial strain depended [68].
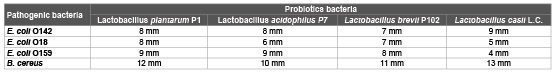
Table 3: Antimicrobial activities of some probiotics on the growth of some isolated strains (diameter of the inhibition zone (mm).
*No inhibition to Salmonella typhimurium and S.aureus
Results recorded in figures 2 and 3 showed that probiotics strains Lactobacillus planterum (p1 ), Lactobacillus acidophilus (p7 ), lactobacillus brevii (p102) and Lactobacillus casii (l.c) reduced E. coli count of 55 × 106 (infective dose) which was artificially inoculated into sterilized skim milk to 9.67 × 104 ± 2.9 × 104 , 10.7 × 104 ± 2.3 × 104 , 9.67 × 104 ± 1.8 × 104 and 8 × 106 ± 1.7 × 106 , with reduction percentage reach to 99.82, 99.8, 99.78 and 85.4 respectively after 24 hrs of incubation at 37°C. Nearly similar results were obtained by Tharmaraj and Shah [69]. While the Control +ve group which inoculated with E.coli only the count increased from 55 × 106 (infective dose) to 10 × 107 ± .5 × 107 at the end of 24 hrs of incubation. The inhibition produced by the probiotic bacteria may be due to the production of organic acids such as lactic, propionic and acetic, hydrogen peroxide, bacteriocins [70].
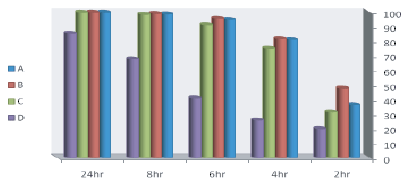
Figure 2: Reduction % of E. coli counts inoculated into sterilized skim milk with lactobacillus strains.
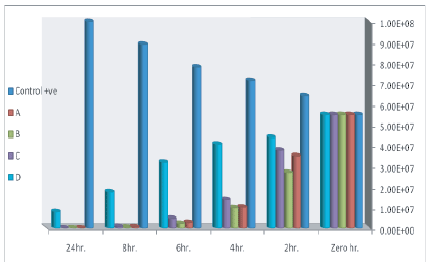
Figure 3: Effect of lactobacillus strains on counts of E. coli inoculated into skim milk samples
Inspection of figure 4 revealed that the pH values of the incubated skim milk samples s decreased till reach to 4.8, 4.9, 4.88 and 4.92 after 24 hrs of incubation at 37°C and this may be due to that probiotic bacteria produce lactic and acetic acids as a metabolic byproducts which play a complementary role in inhibiting pathogenic and spoilage bacteria [71]. pH is an important factor which can dramatically affect bacterial growth, Lactobacillus spp. as a probiotic can tolerate a wide range of pH (1-9) and grow well at acidic pH (1-5) [72]. Probiotics such as Lactobacillus spp. are reported to have inhibitory activity against common human pathogens. They are able to produce antimicrobial substances such as bacteriocins which have great potential to be used in therapeutics and as food bio-preservatives [73].
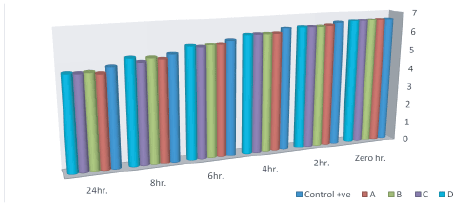
Figure 4: pH of cultured milk
The obtained results in this work give an information that some of examined raw milk, Damietta cheese and Kareish cheese samples sold in Gharbia governorate, Egypt were contaminated with pathogenic microorganisms such as E. coli, Salmonella, S. aureus and B. cereus that indicate inadequate hygienic measures adopted during production, handling, processing, packaging and distribution of the examined milk and its products. Moreover, such organisms may constitute a public health hazard and may lead to food poisoning and/or undesirable changes in the milk products that render them unfit for human consumption and cause economic losses. Probiotics may be added to some milk products as they are valuable to the host by improving the beneficial microbial population, and to improve the quality of such products through control growth of some food borne pathogens.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Elbagory AM, Eman Sh E, Eman KF (2015) Impact of Probiotic Strains on Growth of Some Food Poisoning Bacteria from Milk and Soft Cheese. Nutr Food Technol 1(2): http://dx.doi. org/10.16966/2470-6086.107
Copyright:© 2015 Elbagory AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access