Introduction
The sedentary lifestyle of Western populations is closely associated to
the use of hyper caloric and hyperlipidemic diets, is one of the important
risk factors for Cardiovascular Disease (CVD), the major cause of mortality
in the world.
Western lifestyle and its spread to a growing number of people worldwide,
is increasingly worrying the World Health Organization (WHO) that
recently revealed that ischemic heart disease and cerebrovascular disease
would be in first and second position, respectively, in rank order of deaths
in the frame time from now through the year 2030. Fortunately, CVD,
as well as type 2 diabetes mellitus incidence, cancers and other terrible
diseases, could be prevented with appropriate interventions to reduce the
effects of risk factors. Dyslipidemia represents an important risk factor of
CVD, manifested by elevation or attenuation of plasma concentration of
lipoproteins [1,2]. Dyslipidemia is, therefore, an extremely important
public health problem, involving great cost to both individuals and their
families, as well as representing a huge burden to the health care systems.
Control of triglycerides and cholesterol levels in blood is one of the
most followed practices for preventing Dyslipidemia [3,4]. Statins, the
most therapeutically and commercially successful class of drugs of the
last century, are used to lower blood levels of cholesterol, and PUFAs
(omega-3 polyunsaturated fatty acids) which are mainly fish oil, are widely
used to reduce triglycerides.
Normally, in patients with high cholesterol and triglycerides levels,
doctors prescribe statins and PUFAs at different dosage, evaluated on a
case by case basis, and this is why there are no fixed-dose combinations of
these drugs [5,6]. A second reason is due to technological limitations:
statins are usually poorly soluble and also unstable in fish oil. To obviate
all this, different techniques have been proposed, mainly based on
microencapsulation of statins prior to be mixed in fish oil [7].
Here we report the results of a research project aimed at developing a
technology for the preparation of stable fluid solutions of statins in fish oil.
Statins
The discovery of HMG-CoA (3-hydroxy-3-methylglutaryl-CoA)
reductase, an important enzyme in the metabolic pathway of cholesterol
synthesis, and the statins as its inhibitors, represent a breakthrough in the
prevention of hypercholesterolemia and related diseases. So far this has been
amply demonstrated by clinical evidence [8]. The statins on the market
are fully or partially fermentation-derived (lovastatin, simvastatin and
pravastatin) or entirely synthetic (atorvastatin, rovustatin, pitavastatin
and fluvastatin). All the statins are relatively unstable, and their
degradation is catalyzed by several factors like oxygen, humidity, acidity
and temperature.
The purpose of this paper is not to analyze the different statins
according to their absorption and bioavailability, pharmacokinetic and
metabolism, but to describe statins according to their chemical structure
being responsible of different limitations in the fish oil formulation. Statins
can be grouped into two types: type A (decalin-ring derivatives) and type
B (fluorophenyl derivatives). Another important parameter regards the
equilibrium between the lactone forms (closed, prodrug) or the anionic
carboxylate forms (open; active) (Table 1). With this information in mind
few statins, belonging to both types, were investigated for their solubility
and stability in fish oil solutions.
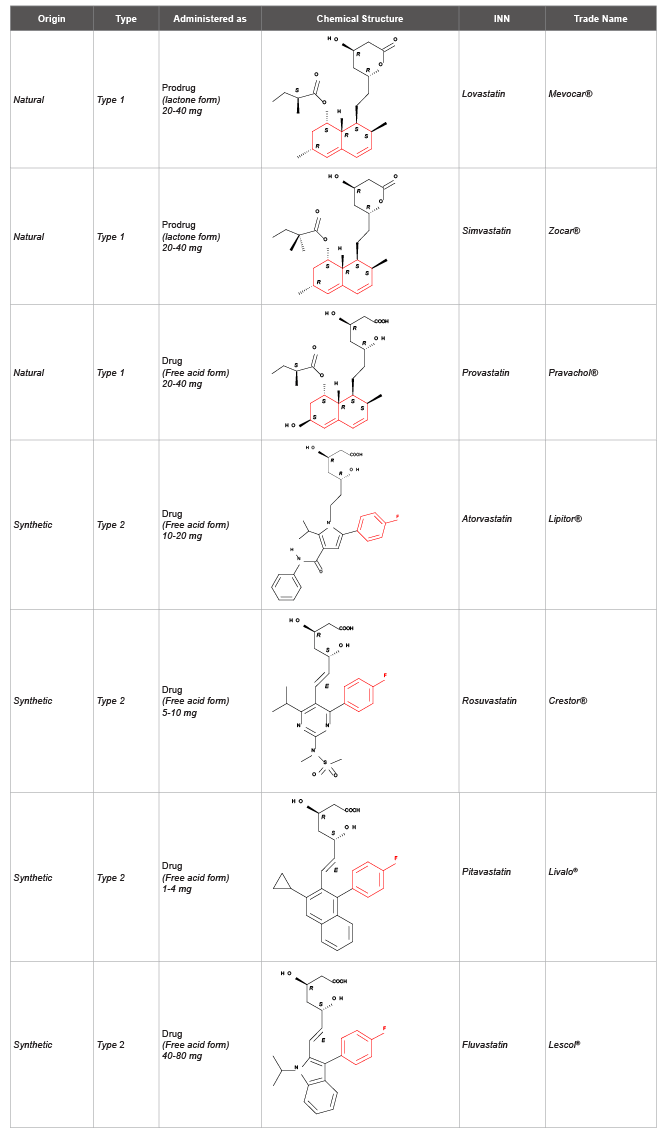
Table 1: Currently available statins on the market, classified according to their chemical structure. INN (International Nonproprietary Names) and trade
name. Typical doses for reduction of LDL (low-density lipoprotein or the “bad cholesterol”) in the range of 25-45%, are also reported.
Fish oil
Marine and freshwater fish oil are the primary sources of omega-3
polyunsaturated fatty acids (n-3 PUFA), composed mainly of DHA
(docosahexaenoic acid) and EPA (eicosapentaenoic acid), which have
been found to regulate lipid metabolism and lower blood triglyceride
levels in a dose-dependent manner, demonstrating beneficial effects in
the prevention of cardiovascular events. According to both primary and
secondary prevention studies, consumption of omega-3 fatty acids, fish,
and fish oil reduces all causes of mortality and various CVD outcomes
such as sudden death, cardiac death, and myocardial infarction, with the
evidence being largely in favor of fish and fish oil supplements [9]. In the
literature it is possible to find many examples where n-3 PUFA from
food or dietary-supplement showed a positive effect in the prevention
and treatment of several diseases, but this is not the topic of this paper.
Interested readers are encouraged to consult recent reviewsto get a broader
understanding of this area [10-12].
Statins in fish oil
Here we report the results of a study on fluid formulations (solutions)
containing n-3 PUFA and statins; although the synergistic effects of statins
associated to PUFA (fish oil) have been documented in several papers [13-
18], it is also known that certain statins are sensitive to acidic environment
causing their degradation to their lactone forms and various isomers.
For example, pravastatin, atorvastatin, and fluvastatin are converted to their
lactone forms in an acidic environment; meanwhile statins in the lactone
form, e.g. lovastatin and simvastatin, are sensitive to alkaline environment
causing this conversion into their acid form. On the other hand, fish oil also
tends to oxidize easily, forcing the use of antioxidants to prevent
rancidity. Taken together, it emerges that combining statin and fish oil
is a particularly difficult task. So, two critical points must be overcome
in order to combine these two active principles, solubility and stability.
Three kinds of formulation have been reported: solutions, suspensions and
solid formulations. However, to protect the components from a possible
degradation, most of them employ techniques of microencapsulation
of at least of one component. Statins are normally poorly soluble in fish oil
and this is associated to their poor bioavailability; with formulations where
they are microencapsulated, further reduction of their bioavailability was
observed. Therefore, to overcome these limitations it might be useful to
have homogeneous solutions. To prepare solutions of statins in fish oil
it is necessary to overcome the limits of their poor solubility, in particular
for the latest generations of statins. In fact, while simvastatin in fish oil has
a solubility of 11mg/ml, atorvastatin (calcium salt) is <0.1 mg/ml soluble.
In both cases, the solubility is far from being suitable to get an
optimal therapeutic dosage. A second drawback to be overcome is the
stability: a solution of simvastatin in fish oil is unstable.
The objective of this study was therefore to identify excipients and/or
a technique to improve the solubility of the statins in fish oil, as well
as their stability. Through a systematic study aimed at testing a set of
emulsifying agents and co-solvents, the best results were obtained with a
purified soybean lecithin, which mainly consists of phosphatidylcholine.
It is very likely that the process of solubilization and stabilization is due
to the formation of reverse micelles. Indeed, the hydrophobic medium in
lecithin is organized to form reverse micelles where the hydrophobic chains
are external and polar residues are internal. These systems can improve
the solubility of the statins. With the simvastatin, more soluble than others,
the main problem to overcome was the stability. The solution to the problem
came with the anhydrification of the solution, on molecular sieves.
Materials and Methods
The omega-3 polyunsaturated fatty acids (n-3 PUFA) are a mixture of
ethyl esters of polyunsaturated fatty acids with content in EPA and DHA
greater than 85%, in a ratio EPA/DHA comprised between 0.9 and 1.5;
product provided by Pronova Norway. The statins used in this study
were all commercially available: atorvastatin as calcium amorphous salt
and simvastatin, by Biocon (India); rosuvastatin as calcium amorphous
salt, by Biocon (India); pitavastatin by MSN Laboratories Pvt. LTD
(India). The polyoxyethylene (20) sorbitan monooleate (Tween® 80)
and the sodium docusate, were purchased from Sigma-Aldrich; the
polyoxyethyleneglyceroltrihydroxy- stearate (Cremophor® RH 40) and the
polyethylene glycol (15) hydroxystearate (Solutol®
HS 15), by BASF Italia
Srl. The mixture of glyceryl and polyethylene glycol esters (Labrasol®
), the
2-(2-ethoxyethoxy)ethanol (Transcutol® P) and the propylene glycol
monolaurate (Lauroglycol® 90), were purchased from Gattefossè Italia,
S.r.l (Milan, Italy). The hydrogenated phosphatidyl choline (Epikuron® 200
SH) and the deoiled lecithin-enriched phosphatidylcholine (Epikuron®
200) used were both furnished by Cargill, and the solid natural lecithin
(Lipoid S PC-3) by Lipoid GmbH.
LC/PDA/MS
A high-performance liquid chromatography (HPLC) instrument
equipped with diode array detection (DAD) combined with mass
spectrometry, was used for the quantification of statins and fish
oil components. The liquid chromatographic apparatus comprised the
following modular components: Alliance system 2695, Photodiode
array Waters 2996 and mass system Waters Quattro Micro (Waters
USA).
LC conditions for simvastatin, atorvastatin, rosuvastatin
The HPLC column was an Intersil®
ODS-3 (4.6 × 250 mm; 5 µm) column,
and the mobile phase used was acetonitrile, water and trifluoroacetic acid
(70:30:0.1, v/v/v). For pitavastatin, acetonitrile, water and trifluoroacetic
acid (30:70:0.1, v/v/v). The flow used was 1ml/min (Table 2).
HPLC method for omega-3 polyunsaturated fatty acids (n-3 PUFA)
The HPLC column was a Waters Symmetry C-18 4.6 × 150 mm 5
µm (Waters USA). The mobile phase consisted of acetonitrile, methanol,
water and trifluoroacetic acid (45:45:10: 0.1, v/v/v/v). The flow used was
1 ml/min. The UV detection and quantitation of EPA and DHA were
performed at λ=215 nm (Figure 1).
Formulations with non-ionic surfactants (Comparative Examples)
Non-ionic surfactants have no charge on their hydrophilic end, which
helps make them superior oily soil emulsifiers. In this study Lauroglycol®
90, Tween® 80, Cremophor® RH40, Labrasol®, and Solutol®HS15 were
used as non-ionic surfactants. The emulsifier was dissolved in n-3 PUFA
and left under mechanical stirring. Then, the statin (i.e., atorvastatin) was
added and the appearance of the solution was evaluated. The emulsifiers
used were all excipients already used in approved pharmaceutical
products, and commercially available. In some cases, a co-surfactant
(i.e., Transcutol® P) was added in a second step to further enhance the
solubility of atorvastatin (Table 3).
Formulations with ionic surfactant-emulsifier (sodium docusate - Epikuron® 200)
These surfactants (cationic or anionic) emulsifier possess a charge on
their polar end. The emulsifier was dissolved directly into the n-3 PUFA
and left under mechanical stirring. Then, the statin (i.e., atorvastatin) was
added and the appearance of the solution was evaluated (Table 3).
With poorly soluble statins (i.e., atorvastatin), a co-surfactant like
Transcutol® P was added in a second step, to further enhance the solubility,
however, without reaching this goal.
Solubility test procedure
For the four statins, atorvastatin, rosuvastatin, pitavastatin and
simvastatin, the maximum concentration reached in fish oil was also
calculated following another procedure: an increasing amount of statin
was added to n-3 PUFA (1 ml) up to a point when a precipitate formed.
Such mixture was left under agitation for 18 hours and then was filtered
on 0.22 µm PTFE filter and analyzed by HPLC, according to the method
reported above (Figure 2). Results are summarized in (Table 4).
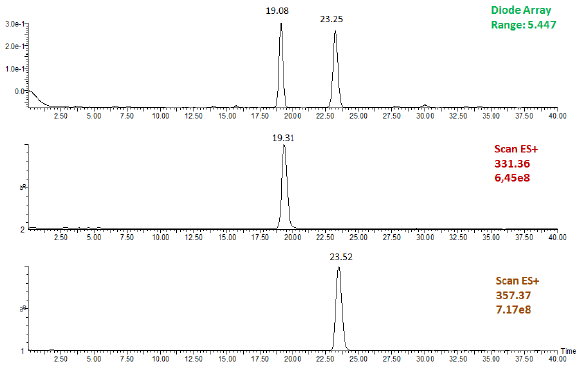
Figure 1: Chromatographic profile of n-3 PUFA analyzed at l=215nm.
a) two main peaks are highlighted: at 19.08 e 23.25 min. b) The first
isolated peaks, corresponding to EPA EE (EPA ethyl ester), with ESMS:
ES+ m/z 331.36; and c) the second to DHA EE (DHA ethyl ester),
with ES-MS: ES+ m/z 357.37.
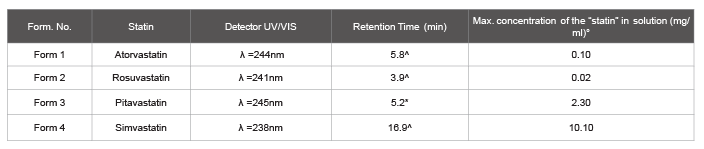
Table 2: HPLC data, with the maximum amount of statins dissolved in n-3 PUFA analyzed. Intersil® ODS-3 (4.6 × 250 mm; 5 µm) column and a solution of CH3 CN/H2 O (70/30 + 0.1% of CF3 COOH) or CH3
CN/H2 O (30/70 + 0.1% of CF3COOH) as eluent, with flow rate at 1ml/min.
^: HPLC methods for simvastatin, atorvastatin, rosuvastatin
*: HPLC method for Pitavastatin
°: Results are the means of a minimum of two independent experiments. ± SD less than 10%.
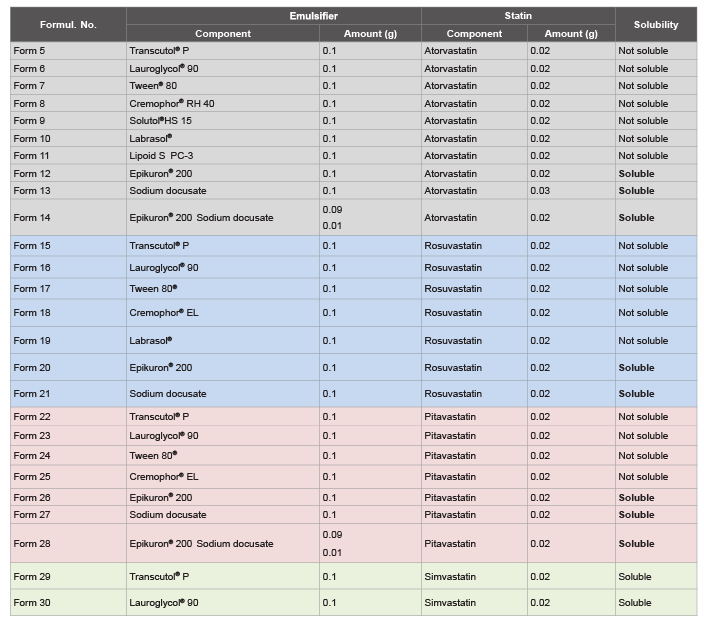
Table 3: Solubility testing of statins (atorvastatin, rosuvastatin, pitavastatin and simvastatin) and fish oil (n-3 PUFA; 0.9 g), in the presence of 10% co-solvent.
Stability tests
For the preliminary stability tests, the samples were placed in a climatic
chamber at 5, 25 and 40ºCand at 60% Relative Humidity (RH). The stability
of the solutions (clear solution from visual inspection) was monitored for
a time up to six months (1st, 3rd and 6th month). A suitable amount of
the fish oil formulated was diluted with methanol and analyzed by HPLC
(Table 5).
To increase the stability
In order to increase the stability, a small amount (10%) of adipic acid
and palmitic acid or citric acid was added to the simvastatin solution.
The presence of said acids did not however enhance the stability
(Table 6).
Drying of fish oil solution with molecular sieves. The molecular sieves
(0.4 nm, beads about 2 mm), were previously activated under vacuum
at 150ºC for 18 hours, then added into the solution and the system was
left overnight without mechanical stirring. The oily solution was finally
obtained by decantation. Stability tests gave surprising results, which are
reported in Table 7.
Results and Discussion
The objective of this study was to prepare homogeneous solutions of
statins in fish oil to overcome the challenge associated with their sparing
solubility and instability.
Different emulsifiers have been tested, demonstrating that with statins
scarcely soluble in fish oil, like atorvastatin, rosuvastatin or pitavastatin,
the addition of ionic emulsifiers, like Epikuron® 200 or sodium docusate,
significantly increases their solubility, reaching concentrations greater than
20 mg/ml (Form 12 and 13; 20 and 21; 26 and 27). A mixture of them
was equally efficient (Form 28). Such concentration are at least ten times
greater than the analogue solutions formulated with non-ionic surfactantsemulsifier
(Tween 80, Cremophor® RH40, Solutol HS 15, Labrasol, etc.),
which in turn do not improve even after addition of a surfactant (i.e.
Transcutol® P, 20%; data not shown) (Tables 3 and 4).
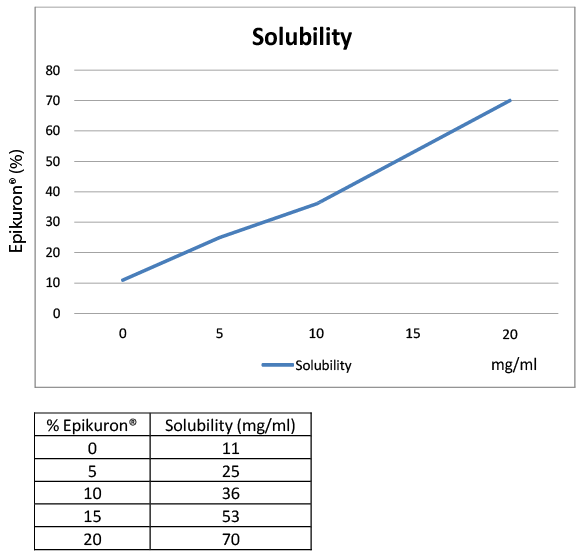
Figure 2: Solubility test of Simvastatin
With more soluble statins, like simvastatin, the effect of ionic and nonionic
surfactant was also verified. The results showed that the maximum
solubility reached with different solvents was in the same range (Form
29-31, 33, 34) (Table 7). With the formulation comprising Epikuron® 200
solubility proportionally increased with the amount of ionic emulsifier
added (see examples in S.I.): with 20% of Epikuron® the solubility
reached 70 mg/ml (Form 32); for the non-ionic solvents, Transcutol® P
and Lauroglycol® 90, the solubility was not increased despite using a
higher amount of solvent (Tables 3 and 4).
From this study, it is clearly demonstrated that to solubilize statins,
above all those poorly soluble in fish oil, ionic emulsifiers, like Epikuron®
200 and sodium docusate, are necessary. With simvastatin, the addition
of 20% Epikuron® greatly increases its solubility up to 70 mg/ml. Even
greater was the effect observed with the latest generation of statins, being
less soluble, where the formulation with ionic emulsifiers increase by up to 2
Log units their solubility: see Form 1 vs. Forms 12 and 13; Form 2 vs. Forms
20 and 21; Form 3 vs. Forms 26 and 27 (Table 4).
In conclusion, the maximum solubility in fish oil of the new statins (e.g.
atorvastatin) is extremely low (<0.1 mg/ml) while with somatostatin higher
solubility has been obtained (~11 mg/ml), however not therapeutically
useful. Here we have clearly demonstrated the feasibility to increase
the solubility of all statins to reach therapeutic dosages (≥ 20 mg/ml), with
stable solutions.
As regards to the chapter of stability, for these new proposed formulations,
two main aspects were monitored: degradation of n-3 PUFA and statins
stability. Fish oil is mainly represented (>85%) by two n-3 PUFA, namely
EPA and DHA, as shown in the chromatographic profile in Figure 1.
In none of the formulations proposed here were the findings of fish
oil degradation significant (Table 5). In the same table, the stability data
of statins, with Epikuron® 200 (Form 12, 20, 26 and 31) or with sodium
docusate (Form 13), monitored at 5º
C, 25ºC and 40°C, up to six months
are showed. At room temperature, for statins in their free acid form,
atorvastatin, rosuvastatin and pitavastatin, the only degradation product
was the closed lactone derivative (10%; ± 3). Otherwise, statins in their
lactone forms, i.e., simvastatin, the only degradation product was the
opened lactone derivative (5%; ± 2). (S.I Figure 1). All statins followed- up
for a month at 5°C showed complete stability. The free acid form statins
in fish oil solution led to only a small percentage of lactone (<5%) (Form
12, 20, 26) (Table 5).
An independent study was done with the simvastatin. In this case
the challenge that had to be overcome was the stability. Simvastatin is
administered as an inactive lactone prodrug: hypothesized that acidic
conditions could stabilize the lactone, citric acid, adipic acid and palmitic
acid, all being excipients commonly used in pharmaceutical formulations,
even in the solid formulations containing simvastatin already available on
the market, which were added to the formulation. However, contrary
to what was expected, in fish oil solutions the presence of a carboxylic or
hydroxy acid did not enhance the stability (Table 6). The only degradation
product observed was the carboxylic acid derivative resulting from the
opening of the lactone ring. Chemically, degradation of lactone can be
explained by the presence of water, so removal of traces of water from the
solution could potentially stabilize the statin. To this end, we proceeded
with anhydrification of the fish oil solution by the addition of suitably
dried molecular sieves. The stability tests gave surprising results (Table 7).
The stability study was then performed with the two co-solvents
individually for a month at various temperatures. The results confirm that
the removal of traces of water from the solution is sufficient to stabilize
simvastatin, regardless of the type of co-solvent used (Table 7). It is
noteworthy that the only anhydrification of the fish oil solution with
activated molecular sieves, it is not enough to stabilize simvastatin and
that only the combination of the two co-solvents (Epikuron® 200 + Sodium
docusate) ensures excellent stability for up to six months. Using
different surfactants such as Transcutol® or Lauroglycol® (formulations
described in the patent literature; WO2006/096806), the resulted solutions,
left under the analogues conditions, were less stable than the ones
described here (Table 7).
The in vivo stability of statins solubilized in fish oil was not the purpose
of this study because it was expected to be similar as when fish oil and statins
are co-administered. However, a preliminary study in simulated biological
fluids (SGF, gastric and SIF, intestinal) with simvastatin has been done.
The results, reported in S.I. (test in simulated biological fluids) showed
that the statin solubilized in fish oil has a stability profile, in both vehicles,
analogous to that reported in the literature.
In conclusion, the approaches here reported were equivalent to that used
for solubilizing drugs in water solutions: indeed, co-solvents like alcohol,
ethylene glycol, Transcutol®, etc. or surfactants, compounds that lower
the surface tension between a liquid and a solid, are among the numerous
approaches available and reported in the literature to enhance the solubility
of poorly water-soluble drugs. In our case, attempting to enhance the
solubility of statins in fish oil co-solvents were investigated with poor
results, as well as emulsifying agents where a few of them, Epikuron®
200, sodium docusate, Tween 80, Solutol HS 15, were able to enhance the
solubility up to 20% p/p. Other emulsifying agents like Cremophor® RH40
and Labrasol were less effective.
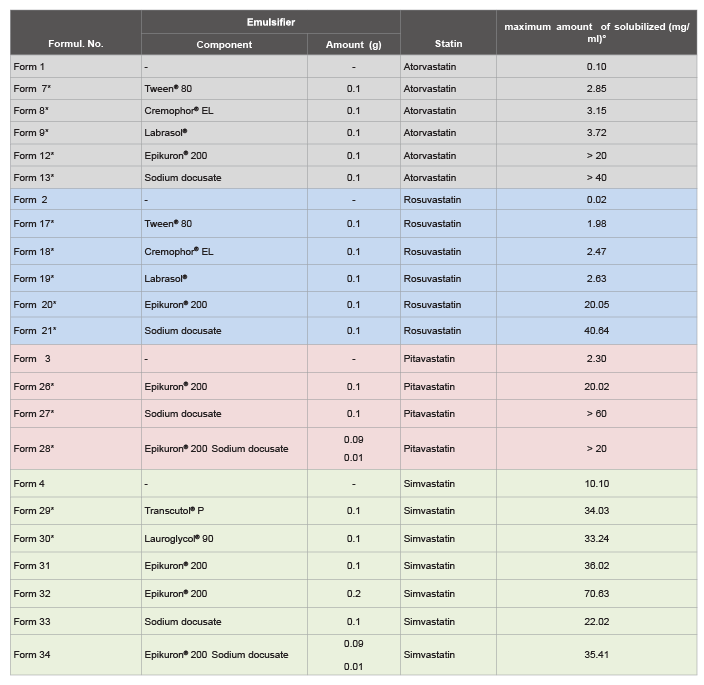
Table 4: Solubility testing: maximum amount of statins (atorvastatin, rosuvastatin, pitavastatin and simvastatin) solubilized (mg/ml) in fish oil (n-3 PUFA; 0.9 g).
(*) The same formulation indicated in Table 3, increasing the amount of statin up to reach its maximum concentration in solution.
(°): Results are the means of a minimum of two independent experiments. ± SD less than 10%.
The physic-chemical mechanisms responsible for enhancing solubility
of the statins in fish oil could be due to the formation of reverse micelles,
as mentioned previously. From an analysis of the various emulsifying
agents here investigated, it was clear that the chemical characteristics of the
surfactant, in particular the polar head, were very important for solubility
of the statins, mainly for those of the new generation (atorvastatin,
pitavastatin, rosuvastatin, fluvastatin). However, such behaviour was also
observed with more soluble statins, such as simvastatin. In conclusion,
to enhancing the solubility of the statins in fish-oil, emulsifying agents
play an important role, participating with their hydrophobic component
in the interaction with the oil molecules and with their hydrophilic
component (head) in the interaction with the statin molecules, forming
reverse micelles. It was also observed that solutions of statins prepared as
their prodrug (lactone form, see simvastatin) once dried, through removed
traces of water, were particularly stable without the need to mix in additives.
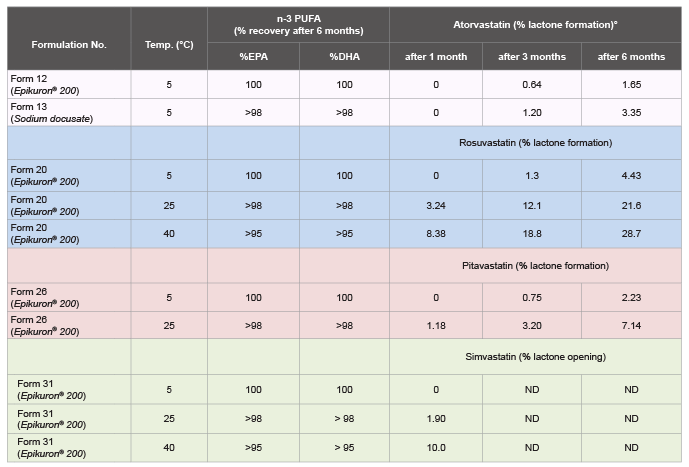
Table 5: Stability testing of statins (atorvastatin, rosuvastatin, pitavastatin and simvastatin), solubilized in n-3 PUFA, up to 6 month storage at 5°C, and at
temperatures up to 40°C (accelerated storage condition).
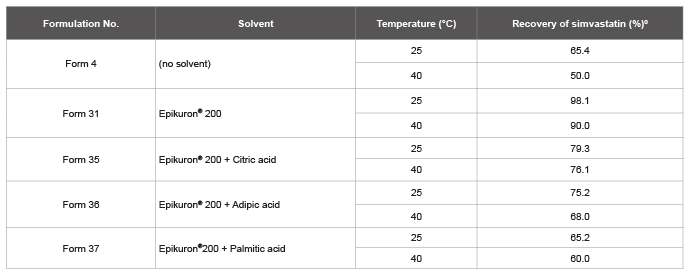
Table 6: Stability testing of simvastatin, solubilized in fish oil, alone or with Epikuron® or Epikuron® and organic acids, after one month at two temperatures,
25 and 40º C.
º: Results are the means of a minimum of two independent experiments. ± SD less than 10%.
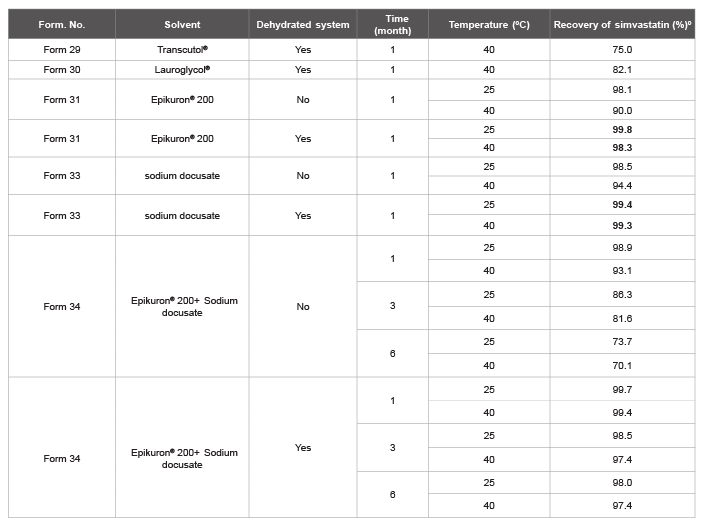
Table 7: Long-term stability testing of simvastatin with Epikuron® or/and sodium docusate, solubilized in fish oil in not dehydrated versus dehydrated solutions.
Transcutol® and Lauroglycol® was also evaluated.
(º): Results are the means of a minimum of two independent experiments. ± SD less than 10%.
Taken together, the experimental data confirms that it is possible to devise
an association between statins and fish oil, through robust formulations,
homogenous and orally administrable, enabling pharmaceutically
exploitable concentrations, in line with therapeutic dosages (Figure 3).
Besides, these solutions are obtainable in short times and with low
costs, rendering the process particularly suitable for industrial
applicability. These formulations may represent a new tool for physicians
and patients in the treatment of pathologies related to hyperlipidemia
and hypertriglyceridemia, hypercholesterolemia, pathologies for which
statins and n-3 PUFA are already prescribed individually in standard
treatment protocols. For example, in patients on a long-term CVD
prevention, taking a single pill could improve the compliance towards
therapy compared to taking the two drugs separately. These results have
been patented [19,20].
Figure 3: Table of Contents (TOC) Art
And last but not least, formulation with sodium docusate, useful
medication against symptomatic treatment of constipation, could be
useful in patients treated with statins, where constipation is a common
adverse effect [21].
Acknowledgment
The authors would like to thank Dr. Gilles Pain for assistance with the
manuscript.
Author Disclosure Statement
The authors claim to work as employees of sigma-tau IFR, S.p.A., an
international pharmaceutical company, which sponsored this research.
Associated Content
* Supporting Information
Chromatographic profile of simvastatin in fish oil solution and their
corresponding degradation product observed; examples of sample
preparation of pharmaceutical formulations; test in simulated biological
fluids (SGF and SIF).
This material is available free of charge via the Internet at ….











