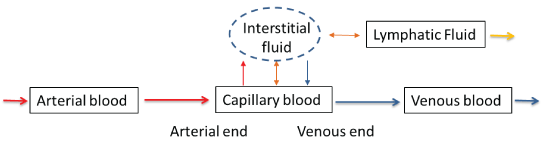
Figure 1: Tissue fluid systems involved in sampling of capillary blood


Wei Huang1* Imelda Omana-Zapata1 Scott J Bornheimer1 Hrair Kirakossian1 Alan H B Wu2 Charlene E Bush-Donovan1
1BD Biosciences, 2350 Qume Drive, San Jose, CA 95131, USA*Corresponding author: Huang W, BD Biosciences, 2350 Qume Drive, San Jose, CA 95131, USA, Tel: 4089542602; E-mail: wei_huang@bd.com
The CD4 cell count is a significant indicator of immune function and remains an important tool to monitor disease progression and predict overall survival in HIV-infected individuals. The gold-standard technology for determining a CD4 cell count is flow cytometry using whole blood collected by venipuncture. Technological advances now allow for the accurate measurement of CD4 cell counts in near-patient platforms, using small sample volumes such as capillary blood from fingerstick samples. To determine whether capillary samples are suitable alternatives to venipuncture samples for CD4 cell count assays, results from paired venous and capillary samples need to be carefully compared.
Literature reports were examined in the context of the physiological differences in sample types, as well as the potential clinical impact of the sampling methods and testing technologies. A trend of approximately 5% positive bias was revealed in CD4 counts from capillary samples compared to venous samples when using the same cell counting technology in adult HIV patients. In practice, this small difference in CD4 cell count is insignificant in most circumstances, and CD4 cell counts obtained from capillary blood samples are equivalent to results from venous blood samples as long as the proper sampling method is followed. Clinicians can now focus on factors related to patient health rather than sample type and testing platform as they use the CD4 cell count to make patient management decisions.
HIV; AIDS; Venous blood; Capillary blood; CD4+ T cell; Point-of-care
Human immunodeficiency virus (HIV) binds to the CD4 glycoprotein on the surface of helper T cells as a part of the mechanism to gain entry into cells and initiate the viral life cycle. Replication of HIV leads to a progressive reduction in the number of host CD4 T-lymphocytes. The CD4 count is an essential parameter in monitoring the immune health of HIV-infected individuals and for prioritizing care and initiation of antiretroviral drug treatment (ART) [1,2]. Of the estimated 36 million individuals living with HIV worldwide, a majority of the population live in developing countries, with sub-Saharan Africa, South and Southeast Asia, and Latin America having the highest prevalence [3].
The global funding response to HIV has led to significant progress in the reduction of morbidity and mortality. As of December 2015, 17 million people living with HIV were accessing ART, up from 15.8 million in June 2015 and 7.5 million in 2010. A continued challenge to broaden access to therapy is the geographically remote areas where many infected reside, and the logistical difficulty of providing them with quality and consistent care. To address this need for expanded care in high burden, resource-limited areas, there is a growing need for rapid, intuitive, and low-cost CD4 count assays that use small-volume samples and are suitable for areas with limited laboratory infrastructure.
Near-patient CD4 testing is a tool to help clinicians stage, baseline, and monitor the immune competency of a patient. In those settings where the ART supply is not continuously reliable or molecular testing is not available, CD4 continues as a methodology to determine eligibility for ART and to monitor the immune health of patients on therapy. Nearpatient CD4 testing has been demonstrated to reduce the time to eligibility assessment and increase retention in care prior to starting treatment in resource-limited settings [4,5]. Recently, several near-patient solutions for CD4 counts have entered the market to address this need, combining lowcomplexity testing with finger-stick capillary blood draws, and bringing quality care to remote geographies.
Diagnostic assays using capillary blood samples are more convenient compared to those requiring venous blood samples, particularly for assays that are performed at high frequency and for point-of-care or near-patient testing. Controversy does exist as to the accuracy and precision of test results obtained from a finger-stick sample compared to a venipuncture sample. For CD4 testing, venous blood is the gold standard for most clinical assays. For capillary blood samples to be accepted as substitutes for venous blood samples, the accuracy and precision of the results need to be understood. Capillary blood samples have been investigated as substitutes for venous/arterial blood samples for measuring blood gas and pH, glucose, and therapeutic drug levels, as well as measuring large polypeptide analytes such as hemoglobin A1C [6-9]. For blood cell counts, there have been several studies comparing white blood cells, lymphocytes, and other cell types in capillary blood vs venous blood. This review will examine blood cell count studies comparing venous to capillary blood samples to provide a context for comparing CD4 cell counts in these two sample types for care of HIV-infected patients. Recent results from nearpatient CD4 counting systems will also be discussed.
Capillary blood obtained by skin puncture is from a dynamic tissue fluidic system that contains circulating capillary blood, interstitial fluid, and lymphatic fluid (Figure 1) [10]. In the arterial end of the capillary, oxygen-rich fluid exits the capillary into the interstitial space, since the hydrostatic pressure is higher than the osmotic pressure. Re-absorption of fluid takes place in the venous end of the capillary as the hydrostatic pressure within the capillary drops. Excess interstitial fluid is collected into the lymph capillary and ultimately returned to the circulation.

Figure 1: Tissue fluid systems involved in sampling of capillary blood
Because of the complexity of the fluidic system involved in the collection of capillary blood, the composition of these blood samples may be more variable and more dependent on the collection techniques than that of the venous blood sample. Standard procedure for the collection of capillary samples requires removal of the first drop of blood while avoiding the application of external pressure around the skin puncture site [11,12]. This is in part to minimize the collection of interstitial and lymphatic fluid into the blood sample. Under ideal blood collection conditions, expert consensus is that a high proportion of capillary blood collected may come from the arterial end of the capillaries due to the higher hydrostatic pressure [13]. This hypothesis is supported by comparing oxygen, pH, and other parameters in arterial, venous, and capillary blood samples. Small but statistically significant differences in blood chemistry testing results have been observed between capillary blood samples and venous blood samples [14-16].
Another aspect of capillary blood collection relevant to blood cell counting results is the cellular response and wound-healing process that takes place after a skin puncture. Key to this wound-healing process is the activation of platelets by thrombin in the blood- clotting cascade, which leads to clot formation, as well as the migration of other cells to the wound site. The impact of these processes on cellular composition during finger -stick sample collection is not well understood, and the significance to cell counts in capillary blood samples is largely unknown. Due to the unique physiological characteristics of capillary blood flow, samples collected by skin puncture may have different properties from venous blood samples. Collection of capillary blood may be affected by multiple factors, including sample collection techniques such as the puncture location and depth, external pressure applied at the wound site, sample volume collected, contamination with particles or debris from the skin, as well as patient demographics and underlying medical conditions [17,18].
It is understood that circulating peripheral lymphocytes represent only a small fraction of the total lymphocyte population in the body, and there is a dynamic recirculating/ migration process between peripheral lymphocytes and lymphocytes that reside in the lymphatic system and other tissues such as spleen, gut, and bone marrow [19]. Blood lymphocyte count, including CD4 cell count, is affected by seasonal, circadian, and medical factors [20-23]. Peripheral lymphocyte counts can also change rapidly with changes in the physiological condition of the donor. For example, a recent study of blood cell counts in elite adolescent swimmers showed over 130% increase in total lymphocyte counts (4,270 vs 1,837 cells/μL) and over 60% increase in CD4+ T-lymphocyte counts (1,152 vs 718 cells/μL) after intensive exercise [24].
Despite these complicating factors affecting cell counts in capillary blood samples, there is a general trend in literature that cell counts for red blood cells and white blood cells are slightly higher in capillary blood samples than in venous samples [25]. A summary of literature studies comparing cell counts in paired capillary and venous samples is shown in Table 1. These studies all share the common feature of using the same cell counting instrument for both sample types [26-33]. With the exception of one study (Hollis et al), all other studies reported higher white cell counts in capillary samples relative to venous samples. Across nine studies, the average bias of the white cell blood count in capillary blood relative to venous blood was 10.1%, with a range of -1.2% to 19.2%. In the Hollis study, a slightly lower white blood cell count was measured in the capillary blood relative to the venous blood samples. The author postulated that the discrepancy with other literature reports could be attributed to the sample collection technique, which required an excessive squeezing of the fingertip to obtain the large volume (~250 μL) of blood required for their study [32].
Table 1: Summary of literature reports comparing blood cell counts in capillar y blood samples vs venous samples
Interest in using capillary blood samples for CD4 cell counts in HIVinfected patients started over two decades ago, since it provides an alternative procedure for easily collecting samples in children and adults with difficult venous access. A few clinical studies have been conducted to compare CD4 cell counts determined in the two different sample types with flow cytometry, and these study results are summarized in Table 2. The earliest study comparing lymphocyte subset cell counts, including CD4+ T-lymphocytes, in capillary and venous samples was reported by Cracknell et al [34]. Cell counts of the major subsets of lymphocytes were assessed by flow cytometry in paired venous and capillary blood samples from 22 children with acute leukemia, and from 10 healthy adults. The results indicated that cell counts were 4.8–6.4% higher in capillary blood samples for cell subsets other than natural killer cells, which were 10.7% higher. Specifically, for CD4+ T lymphocytes, the median cell count was 740 cells/μL with a range of 250-2,500 cells/μL in capillary blood samples, and 710 cells/μL with a range of 230-2,310 cells/μL in venous blood samples. The median CD4 count was 4.2% higher in capillary samples than venous samples, and a p-value of 0.033 was reported for the difference between CD4 counts obtained from paired venous and capillary blood samples.
Table 2: Summary of results f or the stu dies of CD4 cell count s in capillary vsvenous blood samples measured by flow cytometry
The first direct comparison of CD4 cell counts in capillary vs venous samples from HIV- positive patients was reported by MacLennan et al in 2007 [35]. Paired venous and capillary blood samples from 111 consecutive HIV-positive adults at the ART clinic, Queen Elizabeth Central Hospital, Blantyre, Malawi, were tested in parallel for CD4 counts on a flow cytometer. Fingerstick samples were obtained with minimal squeezing of the finger after the first drop of blood was discarded. CD4 counts obtained from capillary samples were higher than CD4 counts obtained from venous samples by an average of 6.6 cells/μL (95% Cl: 1.0- 12.0 cells/μL). When expressed as a percentage of the mean value for each cell type, the biases for the CD4+ lymphocyte, total lymphocyte, and total leucocyte counts were 2.2%, 8.2%, and 15.2%, respectively, for capillary vs venous samples.
A report comparing CD4 counts and % CD4 values in capillary and venous blood samples from HIV-positive adults and children in a resource-limited tropical setting was published by Sitoe et al in 2011 [36]. This cross-sectional study consecutively enrolled 152 HIVpositive patients (101 adult and 51 pediatric patients) attending two outpatient clinics in Maputo City, Mozambique. The enumeration of CD4 cells was performed on two different flow cytometer systems from venous and capillary blood samples within six hours of collection. Using the BD FACSCalibur™ system, the mean absolute CD4+ T-cell counts from capillary and venous blood in adults were 323.4 and 305.1 cells/μL, respectively. The bias based on mean CD4 cell counts in adult capillary vs venous blood samples was 6.3%. On the contrary, the mean absolute CD4+ T-cell counts obtained in capillary and venous blood in children were 1,262.7 and 1,335.8 cells/μL, respectively, and the bias based on mean CD4 cell counts for capillary vs venous blood samples was -5.3%.
When the same set of samples was tested on the BD FACSCount™ system, the bias based on mean cell counts for capillary vs venous samples was 1.7% in adults and -5.8% in pediatric patients. This was the first report in which the pediatric patient CD4 cell count results were analyzed separately when capillary and venous blood samples were compared. The median age for pediatric patients in this study was 4 years, with a range of 4 months to 15 years, and the negative bias trending of CD4 counts for capillary blood samples observed in pediatric patient samples was opposite to what has been observed in adult patient samples.
In the last few years, several point-of-care or near-patient devices for the CD4 counting assay have been developed and commercialized, including the Alere Pima™ system, BD FACSPresto™ system, Partec CyFlow® miniPOC system, and the HumaCount CD4 now system [37,38]. These devices were designed for applications in resource-limited settings and generally do not require cold-chain storage, extensive instrument maintenance, or user training. Moreover, most of these systems are capable of performing the CD4 cell count assay using both venous and capillary blood samples and require smaller volumes of whole blood than standard flow cytometer-based methods. Among these CD4 count systems designed for resource-limited settings, the Alere Pima system and the BD FACSPresto system have received WHO prequalification. A search on PubMed using the key words “PIMA CD4” showed 38 publications, including a recent meta-analysis of the performance of the system in 22 independent studies [39]. Among these publications, four studies included direct comparison of CD4 counts in paired venous and capillary blood samples using the Pima system. A similar search for the BD FACSPresto system yielded four publications, with three studies that included direct comparison of CD4 counts in paired venous and capillary blood samples. Results from these seven publications comparing CD4 counts in paired venous and capillary blood samples using two point-of-care/near-patient devices are summarized in Table 3.
Table 3: Summary of results for the studies of CD4 cell counts in capillary vs venous blood samples measured with near-patient devices
*Data collected from Antwerp, Belgium site.
**Data collected from the Da res Salam, Tanzania site
***Some of the 95% confidence intervals were not reported in the original publication and were calculated based on the reported limits of agreement in the
Bland-Altman Analysis and the sample size
The Alere Pima system uses a microfluidic cartridge that requires a peristaltic pumping system for moving the whole blood within the cartridge and for mixing of the staining reagent with cells. Cell detection is achieved with a fluorescence imaging system. The throughput is about three measurments per hour for each instrument, and only the absolute CD4 cell count result is produced. A perfomance evaluation of the Pima system in field tests in South Africa was reported in 2012, which included the comparison of CD4 cell counting results using capillary vs venous blood samples [40]. The comparision of CD4 count results in capillary vs venous samples using the Pima system was inconclusive (capillary vs venous bias ± standard deviation = -18.81 ± 162.2 CD4/μL, N = 77). The 95% confidence interval of the bias was -55 to 17 CD4 cells/μL (calculated based on reported data). In a field settting study of 175 patients using the Pima CD4 system in India, a mean bias of 2.8% was observed in capillary blood samples vs venous blood samples [41].
When capillary blood samples from 840 HIV patients in Kenya were compared with venous blood samples using the Pima system, a mean bias of 7.7 CD4 cells/μL was observed (95% confidence interval of the mean bias was -0.2 to 15.6 CD4 cells/μL, calculated based on a sample size of 840 and a standard deviation of 116.53) [42]. Finally, in a WHO multicenter evaluation of CD4 count assays, a mean bias of -5.5% was observed in capillary blood samples vs venous blood samples in Antwerp, Belgium (N=240) and a mean bias of 8.4% was observed in Dar es Salam, Tanzania (N=200) [43]. It was reported that in Antwerp, nurses had to squeeze the fingers of some patients to get a good capillary blood drop, and this might have caused dilution of blood samples and lower cell counts. In addition, some patients experienced heavy finger bleeding after fingerprick, resulting in either incorrect filling of cartridges or blood dripping.
Compared to the Pima system, the BD FACSPresto system provides not only absolute CD4 cell counts, but also %CD4 (% of CD4+ T-lymphocyte count in total lymphocyte count) values and hemoglobin measurements in the same assay run [44-48]. This system has a throughput of 10 sample runs per hour for each instrument. The minimum sample volume for CD4 cell counts is approximately 25 μL, and the system is designed to test CD4 cell count using both venous and capillary blood samples.
A performance evaluation of the BD FACSPresto system in both laboratory and typical field clinic settings in South Africa was reported by Coetzee et al in 2016 [45]. Phase I of the study was performed in a laboratory setting to assess the baseline accuracy and precision of the BD FACSPresto system with remnant venous blood samples obtained from a CD4 testing laboratory. Phase II of the study was conducted at the Witkoppen HIV counseling and testing clinic in Johannesburg, and the age of the patient population was 37.4 ± 10.8 years. Cartridges were filled with capillary blood samples from a fingerstick using a BD Vacutainer® 1.5-mm blade with 2-mm depth per the manufacturer’s instructions. Venous blood samples were collected in EDTA blood tubes and manually filled into cartridges. For the 118 paired capillary and venous samples, the average Bland-Altman bias for CD4 cell counts was 50.2 ± 92.79 cells/μL and the bias for %CD4 values was -2.8 ± 2.73 percentage points. Using the dataset in the supplement of the publication, the calculated mean BlandAltman bias for CD4 cell counts in capillary vs venous blood samples was 8.6% with a 95% confidence interval of 5.4%-11.9%. The calculated mean bias for %CD4 values was -12.7% with a 95% confidence interval of -15.5%– -9.9%.
Angira et al [46] reported results of a clinical evaluation of the BD FACSPresto in testing CD4 counts in AIDS patients in Kenya. The performance of accuracy, precision, stability, and linearity for CD4, %CD4, and hemoglobin assays using both capillary and venous blood samples was reported. For the comparison of venous and capillary samples, paired venous and capillary samples from 162 HIV-positive patients were collected and tested. The average age of patients was 31.2 with a range of 2-77 years old. Thirty-three (33) patients were classified as children (range 2-17 years old), and 129 patients were classified as adults (range 18-77 years old). The data set provided in the supplement of the publication was further analyzed for the comparison of CD4 count results in capillary and venous samples.
In the combined population (N=162), a mean bias of 6.6% was observed in the CD4 cell count results with a 95% confidence interval of 4.9%–8.3%. The %CD4 value measured in capillary blood sample had a mean bias of -3.3% with a 95% confidence interval of -5.1%– -1.5%. The hemoglobin concentrations measured from capillary blood samples had a mean bias of 2.9% with a 95% confidence interval of 2.2%-3.6%. In the pediatric population with the age range of 2-17 years old (N=33), a mean bias of 3.3% was observed for CD4 cell counts measured from capillary samples vs venous samples, and the 95% confidence interval was 0.3%-6.2%. In the adult population with the age range of 18-77 years old (N=129), a higher bias of 8.6% was observed, with a 95% confidence interval of 6.7%-10.4%. Deming regression and Bland-Altman graphs comparing CD4 counts obtained from capillary and venous samples are shown in Figure 2. The CD4 counts measured in capillary samples had a good correlation with CD4 counts measured in paired venous samples, and the R2 value was 0.97 based on linear regression.
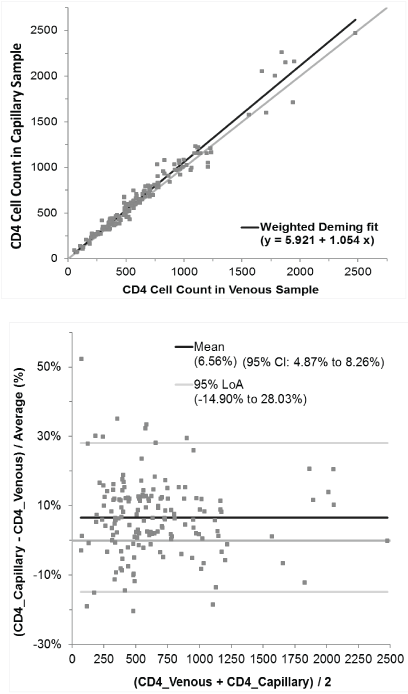
Figure 2: Deming regression and Bland-Altman graphs comparing CD4 cell count results in capillary and venous samples measured with BD FACSPresto (N=162)
A WHO prequalification performance evaluation of the FACSPresto system was recently published [47]. This study was conducted at the Institute of Tropical Medicine in Antwerp (Belgium) and the Infectious Disease Clinic in Dar es Salaam (Tanzania), using both venous and capillary blood samples collected from adult HIV patients. The study concluded that the BD FACSPresto system performed well using both venous and capillary blood. The absolute CD4 counts from venous samples, as compared to capillary samples, showed a better correlation with the reference flow cytometry method and a lower bias. The average bias from the Antwerp site was 3.1% with a 95% confidence interval of 0.7% to 5.5%, and the average bias from the Dar es Salam site was 11% with a 95% confidence interval of 8.8% to 13.2%. The absolute CD4 counts obtained from venous and capillary samples showed an excellent correlation at both study sites with a reported R value of 0.97 and 0.98, respectively.
In a recently published side-by-side comparison of the performance of the BD FACSPresto system with the Pima system in the CD4 assay, the BD FACSPresto system showed a precision that was similar to the standard flow cytometry system, and the reported coefficients of repeatability on the BD FACSCalibur, BD FACSPresto, and Pima were 4.13%, 5.29%, and 9.79%, respectively [48]. The high precision of the BD FACSPresto system may be the reason that the small difference in the CD4 cell counts obtained from capillary samples vs venous samples has been observed consistently with statistical significance.
As increasing numbers of HIV-infected individuals living in resourcelimited areas of the world are expected to gain access to antiretroviral treatments, there is a continued need for CD4 cell counting assays to assess the immune health of patients. WHO guidelines [49,50] advocate for the continued importance of CD4 testing for assessing baseline disease progression, starting and stopping prophylaxis for opportunistic infections, and in setting priority for patient treatment when universal test-and-treat is not available. Recent health economics analyses demonstrated the value for point-of-care CD4 testing even in relation to test-and-treat [51,52 ]. Use of capillary blood samples for the CD4 assays is compatible with point-of-care testing because of the reduced operational complexity, rapid turnaround for results, lower cost and acceptability to patients [53]. Understanding the correlation between CD4 count results obtained from capillary samples and venipuncture samples is critical for the adoption of these near-patient CD4 count assays. Although there are physiological differences between blood samples obtained by fingerstick and venipuncture, a review of the literature revealed only a small positive bias in total white blood cell and CD4 cell counts in capillary blood samples compared to venous blood samples when using validated methods and testing platforms. A recent study concluded that CD4 bias should not exceed +/- 50 cells/µL in order to ensure that patients are appropriately classified or prioritized for clinical therapeutic decision making [54]. In this review, it was demonstrated that the bias between capillary and venous CD4 cell counts across many studies was
Download Provisional PDF Here
Article Type: Review Article
Citation: Huang W, Omana-Zapata I, Bornheimer SJ, Kirakossian H, Wu AHB, et al. (2017) CD4 Counts in Capillary and Venous Blood Samples. J HIV AIDS 3(2): doi: http://dx.doi.org/10.16966/2380-5536.138
Copyright:© 2017 Huang W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access