
Figure 1: HIV care continuum among male sex workers (MSWs), n=210, Lima, Peru, 2014-15


Angela M Bayer1,3* Chanelle M Díaz2 Marina Chiappe3 Amira N Baker1 Miguel S Egoavil3 José E Pérez-Lu3 Pamina M Gorbach4 Patricia J García3
1David Geffen School of Medicine, University of California, Los Angeles, USA*Corresponding author: Angela M Bayer, David Geffen School of Medicine, University of California, Los Angeles, 10833 LeConte Ave, CHS 12-105, Los Angeles, CA 90095 and School of Public Health and Administration, Universidad Peruana Cayetano Heredia, Lima, Peru, USA, E-mail: angelabayerx@gmail.com
Background: In Peru, there is little information about the HIV care continuum. Therefore, we conducted a study to describe the HIV care continuum among male sex workers (MSWs) in Lima.
Methods: We applied close-ended surveys with 209 MSWs about their engagement in the HIV care continuum and open-ended surveys with 34 MSWs who are people living with HIV (PLHIV) to explore their linkage to and retention in HIV care.
Results: Of 209 MSWs, only 71% (n=148) reported a lifetime HIV test and 56% (n=116) of all MSWs received confirmatory HIV test results. Of the 34 MSWs who are PLHIV, 76% had received HIV care at least once, but only 59% were currently in care, 50% were currently on ART and an even lower 32% had been on ART for 6 months or more. The primary reason for non-linkage to HIV care is the multiple visits to link at the Ministry of Health (MOH). Remaining in care was also challenging, particularly at non-governmental organizations (NGOs).
Conclusions: Substantial barriers linking to and remaining in HIV care result in significant delays in linkage and high proportions of PLHIV that remain unlinked or become unstably linked following initial linkage. Urgent implementation science research is needed to facilitate linkage to HIV care and strengthen retention in HIV care post-linkage.
Male sex workers; HIV care
Early initiation of antiretroviral therapy (ART) has been shown to improve individual health outcomes of people living with HIV (PLHIV) and decrease HIV transmission, with the potential to change HIV incidence at the population level [1-5]. However, early ART initiation requires early HIV diagnosis and rapid linkage to HIV care. In many resource constrained settings, HIV care consists of pre-ART care and, when indicated according to national guidelines, ART.
In Peru, the HIV epidemic is concentrated among men who have sex with men (MSM) and transwomen (TW), including a key sub-group of MSM, male sex workers (MSWs). HIV prevalence is very low among general population males and females (0.5%) [6] and female sex workers (1%) [7,8]. By contrast, HIV prevalence is much higher among MSM and TW. HIV prevalence is 11% among MSM and TW [9] and is even higher among MSWs, 23% [10]. MSWs, especially lower-income MSWs in the downtown area of Peru’s capital city Lima, are at increased risk of HIV given higher vulnerabilities and risk behaviors. Lower-income MSWs reported that they engage in survival sex work that consumes most of their daily lives and results in low earnings and an inability to take steps toward achieving life goals and, for those that wish to, exit sex work. They perceive high HIV risk and vulnerability but, given lack of agency, they also perceive significant challenges to using condoms [11]. This results in low consistent condom use with recent anal sex: only 38% of lowerincome MSWs always used a condom with insertive anal sex and only 40% always did so with receptive anal sex [10].
Data on the continuum of HIV care in Peru is limited and based primarily on estimates. The literature describes the HIV care continuum as a fluid process that moves from 1) diagnosed with HIV to 2) linked to HIV care to 3) retained in HIV care to 4) in need of ART to 5) on ART to 6) virally suppressed [12]. Estimates show that of the 72,000-76,000 Peruvians aged 15 and older living with HIV, only 30-36% of all PLHIV and 24% of MSM and TW are receiving ART [13-15]. Despite their increased risk for acquiring HIV [16], data on the HIV care continuum among MSWs is completely lacking in Peru and globally [17].
Early, rapid linkage to and retention in HIV care are critical for individual PLHIV and for health systems globally and particularly in lowand middle-income countries (LMICs) and other resource-constrained settings. The World Health Organization (WHO) recently shifted its recommendations to encourage early initiation of ART regardless of the CD4+ T cell count [18]. The Peruvian National Guidelines for the Comprehensive Care of Adults with HIV at the time of the study recommended the following for initiation of ART: among PLHIV with a CD4+ T cell count of ≤ 350 through December 13, 2014 [19]; and among PLHIV with a CD4+ T cell count of ≤ 500 from December 14, 2014 forward [20]. Importantly, under both versions of the guideline, PLHIV in Peru are only able to initiate ART after completing a series of visits and tests that are mandated by the guidelines and that we explore and describe in this study.
Early ART initiation may improve early retention in care [21]. However, delays linking to HIV care after diagnosis are common and late presentation for care remains the norm in many settings, particularly in Latin America where most patients have a CD4+ T cell count of <200 at ART initiation [12,22]. Patients who enter care late in their HIV disease have substantially higher treatment costs than those who enter earlier [23]. Long-term retention in HIV care is critical, as poor retention has individual- and population-level implications. Poor retention in HIV care is associated with delayed ART initiation, suboptimal ART adherence and detectable viral loads [24-26]. Interruptions in care are associated with HIV-related hospitalizations and increased mortality [25,27-29]. Retention in care has been linked to reduced sexual risk behaviors among PLHIV [30]. At the community level and notably, early retention in HIV care is associated with a decreased cumulative viral load burden [31], which may explain why early treatment initiation has been shown to reduce HIV transmission by greater than 90% [5].
In Peru, there is little information about the HIV care continuum, particularly about linkage to and retention in care, in the overall population or sub-groups. We conducted a mixed methods study to describe access to the HIV care continuum and provide an in-depth characterization of linkage to and retention in HIV care among MSWs in Lima, Peru.
Data was collected in Lima and neighboring Callao, which are home to 9.8 million or 30% of Peru’s 32.8 million inhabitants. HIV/AIDS in Peru is also concentrated in Lima/Callao, representing 62% of HIV cases and 72% of AIDS cases reported nationally from 1983-2012 [13].
Participants are from a study to estimate the initial effectiveness of a multi-component skill-building intervention (the “El Punto” community center) to prevent HIV among MSWs. The manuscript to describe and evaluate the intervention is forthcoming. A total of 209 MSWs in Lima participated in this study. Participants were recruited from MSW sex work venues identified during an earlier ethnographic mapping carried out by our study team [32]. Peer recruiters, who are MSM who have extensive experience working with both MSM and MSWs, revisited street-based venues and invited MSWs to participate. Venues included: plazas and parks; streets; and bars and clubs. Eligibility criteria included: 1) born male and self-identified as male (non-transgender); 2) had traded sex for money, goods, or services at least once in the previous week; 3) resided in Lima; 4) age 15 or older; 5) able and willing to provide informed consent. Transwomen were excluded from the study since MSWs in formative research specified that they wanted a community center open only to maleidentified sex workers and since in Lima, there are other interventions and services to serve transwomen and transwomen sex workers.
The study had three phases: 1) a baseline assessment with a behavioral survey and testing for HIV and other sexually transmitted infections (STIs); 2) the “El Punto” community center intervention that included multiple services such as a drop-in center, facilities to cook and wash clothes, a space to rest, and different types of individual and group personal development opportunities; and 3) an endpoint assessment that re-applied the behavioral survey and HIV/STI testing to estimate the effectiveness of the intervention. The study was carried out from January 2014 to August 2015.
One of the services provided through the community center was support and accompaniment to link to HIV care for MSW participants who are PLHIV. The larger study did not originally include this service. However, we included it after observing the significant percentage of participants living with HIV who were not linked to HIV care. More detail is provided in section 2, immediately below.
Close-ended surveys about engagement in the HIV care continuum among all MSWs: During the baseline assessment, we asked the 209 participants about their engagement in HIV care, including whether they had 1) ever received an HIV screening test and 2) received their confirmatory test result. Data was collected through computer-assisted personal interviewing (CAPI) programmed with Epi Info 7 (Centers for Disease Control and Prevention, Atlanta, GA, USA). This information is presented in Results 1, table 1 and figure 1.
|
n=209 n (%) |
Age in years, mean (standard deviation) |
25.6 (6.2) |
High school graduate |
122 (58%) |
Stable living (pays monthly rent) |
58 (28%) |
Sexual orientation |
|
Bisexual |
120 (58%) |
Heterosexual |
50 (24%) |
Homosexual |
38 (18%) |
Years in sex work, median (interquartile range - IQR) |
4.7 (2.1-9.4) |
Number of clients in past 3 months, median (IQR) |
15 (8-37) |
Any condomless anal intercourse in past 3 months |
89 (43%) |
HIV positive |
51 (24%) |
Table 1: Male sex workers’ socio-demographic and sex work related characteristics, n=209, Lima, Peru, 2014-15
During the baseline assessment, all participants were tested for HIV through rapid HIV testing using a finger-stick blood specimen (Bioline HIV 1/2 3.0, Standard Diagnostics, Korea) followed by confirmatory testing with HIV-1 antibody immunofluorescence assay (IFA) for reactive rapid tests.
Open-ended surveys about engagement in the HIV care continuum among MSWs living with HIV: During the skill-building intervention, one service for participants already living with HIV or diagnosed with HIV at baseline was support linking to HIV care. Depending on each participant’s preference, this included: 1) education and motivation about the importance of professional HIV care and of caring for one’s general and HIV-related health, through individual counseling in-person, by phone and on Facebook; 2) orientation to link to HIV care, focused on the options (non-governmental organizations/NGOs, Ministry of Health/ MINSA), advantages and disadvantages of each option (e.g., waiting times, cost) and the linkage process, through in-person individual counseling; and 3) accompaniment to link to HIV care, by accompanying the person to the healthcare establishments for “linkage visits,” motivating the person to continue the process and covering miscellaneous costs such as transportation. “Linkage visits,” which include the multiple visits and tests needed to gain entrance to HIV care in Peru, are described in the case studies below. Participants in this study received support and accompaniment for as long as they desired or until they achieved linkage.
While supporting participants in linking to HIV care, we became aware of the complexities of linkage to and retention in HIV care. Therefore, in the endpoint assessment, we added open-ended survey questions about the HIV care continuum for the 34 participants who were diagnosed with HIV prior to that time point and returned for the endpoint assessment. Interviewers used a paper-based instrument to ask participants about their engagement in the HIV care continuum from diagnosis to present. After asking about whether they had ever received HIV care, we asked about the following for each HIV care interaction: 1) institution, 2) type of care received (attempt at linkage, HIV care, ART), 3) time in care, and 4) time on ART. We defined “stable linkage” as 6 or more months in the same health establishment and “unstable linkage” as less than 6 months in the same health establishment. We defined “retained in care” as 6 or more months at the same health establishment following the “stable linkage” closest to the assessment time point. Finally, we defined “unlinked” as having no HIV care post-diagnosis. Among those that linked in the last 6 months of the endpoint assessment, this time could be shorter when we did not have a sufficiently long follow-up. This information is presented in Results 2 and 3, table 2 and figure 2.
|
Participants with stable linkage to HIV care, n=20 |
Participants with unstable linkage post-diagnosis, n=6 |
Participants with no care post- diagnosis, n=8 |
||||
Care at NGOs only, n=7 |
Care at MINSA only, n=8 |
Care at NGOs, then MINSA, n=5 |
|||||
First reported HIV diagnosis during our study, n (%) |
0 (0%) |
6 (75%) |
2 (40%) |
2 (33%) |
7 (88%) |
||
Already linked to stable HIV care pre-study, n (%) |
6 (86%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
||
Support for linkage process from EI Punto community center study |
|||||||
Education/motivation to link, n(%) |
0 (0%) |
0 (0%) |
0 (0%) |
2 (33%) |
8 (100%) |
||
Orientation to link, n (%) |
1 (14%) |
5 (63%) |
2 (40%) |
2 (33%) |
0 (0%) |
||
Accompaniment to link: 1-3 visits, n (%) |
0 (0%) |
2 (25%) |
1 (20%) |
2 (33%) |
0 (0%) |
||
Accompaniment to link: all (10+) visits, n (%) |
0 (0%) |
1 (13%) |
2 (40%) |
0 (0%) |
0 (0%) |
||
HIV diagnosis (dx) to stable linkage to HIV care (if stably linked) or data collection (if unstably linked): delays in linkage and periods without HIV care |
|||||||
# of months from 1) dx to first stable linkage if stably linked |
0.0 (0.0-6.0) |
13.5 (6.0-15.8) |
23.0 (14.0-70.0) |
38.5 (18.5-105.0) |
12.0 (11.8-13.3) |
||
Range |
0.0-166.0 |
4.0-24.0 |
5.0-102.0 |
16.0-121.0 |
10.0-33.0 |
||
# of months with no HIV care between dx and 1) stable linkage or 2) data collection: median (IQR) |
0.0 (0.0-6.0) |
5.5 (1.8-13.0) |
17.0 (19.0-61.0) |
17.0 (13.0-56.3) |
12.0 (11.8-13.3) |
||
Range |
0.0-162.0 |
0.0-21.0 |
0.0-88.0 |
11.0-119.0 |
10.0-33.0 |
||
HIV dx to stable linkage to HIV care: any care and time in care prior to linkage (NGOs) or time to link to care (MINSA) |
|||||||
AnycareatNGOsprior to stable linkage, n (%) |
1 (20%) |
0 (0%) |
6 (100%) |
4 (67%) |
NA |
||
# of months in care at NGOs during linkage: median (IQR) |
0.0 (0.0-0.0) |
NA |
2.0 (1.0-4.0) |
1.5 (0.3-2.0) |
NA |
||
Range |
0.0-4.0 |
|
1.0-10.0 |
0.0-43.0 |
NA |
||
# of months to link to care at MINSA: median (IQR) |
NA |
3.5 (3.0-4.3) |
4.0 (4.0-5.0) |
NA |
NA |
||
Range |
|
2.0-13.0 |
3.0-5.0 |
NA |
NA |
||
Retention in HIV care post-stable linkage: time since stable linkage, changes in care institution or finance regimen, and periods without HIV care |
|||||||
# of months from stable linkage to data collection: median (IQR) |
24.0 (23.0-51.0) |
3.5 (1.8-7.0) |
6.0 (6.0-7.0) |
NA |
NA |
||
Range |
18.0-78.0 |
0.0-11.0 |
4.0-12.0 |
NA |
NA |
||
1+ changes in institution or finance regimen at same |
6 (86%) |
0 (0%) |
0 (0%) |
NA |
NA |
||
# of changes to new institution/finance regimen: median |
1.0 (1.0-2.5) |
0.0 (0.0-0.0) |
0.0 (0.0-0.0) |
NA |
NA |
||
Range |
0.0-4.0 |
0.0-0.0 |
0.0-0.0 |
NA |
NA |
||
Table 2: Process for linkage to HIV care and retention in care post-linkage at non-governmental organizations (NGOs) and Ministry of Health (MINSA) among MSWs living with HIV, n=34, Lima, Peru, 2015
Case studies of linkage to HIV care: While accompanying PLHIV participants when linking to care, we carried out two in-depth case studies of the process using observation to document each visit step-by-step and the entire process visit-by-visit for two participants at two hospitals. One hospital is among the top three in number of PLHIV served in Lima (over 2,000 PLHIV) and one is among the top 20 (about 400 PLHIV) [33]. This information is presented in Results 3 and table 3.
Surveys about engagement in the HIV care continuum: Close-ended questions were analyzed descriptively using STATA 13.0 (StataCorp LP, College Station, Texas, USA). Open-ended questions were synthesized in Excel 2011 (Microsoft Corp. Redmond, WA, USA), which was also used for descriptive analyses.
Observations of linkage to HIV care: Data was synthesized in Word 2011 and summarized in flow charts in PowerPoint 2011 (Microsoft Corp.).
The study protocol was reviewed and approved by the ethical review boards of the University of California, Los Angeles and the Universidad Peruana Cayetano Heredia. All study participants provided verbal informed consent prior to commencing study participation.
The socio-demographic and sex work related characteristics of the 209 participants are described in table 1.
There are several drop-offs in the HIV care continuum (Figure 1),which we depict using a figure based on the HIV and Transitions Framework proposed by Powers and Miller [34].
Two hundred nine MSWs provided information about their engagement in the HIV care continuum during the baseline assessment. The first dropoff is in HIV screening, with lifetime HIV screening reported by 71% (n=148) of MSWs. The second drop-off is between the HIV screening test and receipt of the screening and/or confirmatory test result, with only 56% (n=116) of all participants reporting receipt of at least one HIV test result in their lifetime.
During the baseline, 51 of 209 MSWs (24%) tested positive for HIV. Of these 51 PLHIV, 34 returned for the endpoint assessment. Among the 34 PLHIV, there were drop-offs throughout linkage to and retention in HIV care (Figure 1). About three-fourths (76% or 26) of MSWs living with HIV reported at least one HIV care visit post-diagnosis. Only 59% (n=20) were currently linked to care and only 50% (n=17) were currently on ART. An even lower 32% (n=11) had been on ART for 6 continuous months or more.

Figure 1: HIV care continuum among male sex workers (MSWs), n=210, Lima, Peru, 2014-15
The 34 PLHIV in the endpoint assessment provided in-depth information about their linkage to and retention in HIV care, from diagnosis to present.
Of the 34 PLHIV, half (n=17) reported being diagnosed with HIV for the first time during our study (Table 2). Of the 17 PLHIV with a previous diagnosis, only 6 (35%) were stably linked to HIV care at baseline, although an additional 7 (41%) had received HIV care at one or more time points post-diagnosis. During our study, we supported linkage to HIV care for all 28 unlinked PLHIV, providing: education for 10 people; orientation for 10 people; and accompaniment during linkage for 8 people (Table 2), depending on each participant’s preferences. Of the 28 PLHIV who were unlinked at baseline, 14 PLHIV stably linked to HIV care during the intervention period, 6 PLHIV were unstably linked since they had received some HIV care post-diagnosis, and 8 PLHIV remained unlinked since they had received no HIV care post-diagnosis. Of the 6 unstably linked PLHIV, 2 were in the midst of the linkage process at the close of the endpoint assessment. One was diagnosed in our study, had reached visit 10 of a total of 12 linkage visits, and was waiting for lab results to complete the process (see table 3 on linkage process). One, who had received previous long-term HIV care post-diagnosis and become unstably linked, was delaying a new linkage process due to a tuberculosis (TB) diagnosis during linkage and the time needed for daily TB treatment (see figure 2, participant 6). The 8 PLHIV who reported no HIV care received education and motivation to link to care from our study, but did not want to initiate care, including the 1 person who was diagnosed prior to our study.
PLHIV in our study received their HIV care according to one of three models: 1) only at NGOs – through a research study (with monetary incentives), paid care or free care, 2) only at MINSA, or 3) at NGOs and MINSA (Table 2). Among the 20 PLHIV who were stably linked to HIV care, the time from HIV diagnosis to stable linkage and the linkage process itself varied by the type of institution (NGO versus MINSA). Those who sought care solely at NGOs linked more quickly (median 0.0 months, interquartile range (IQR) 0.0-6.0 months) than those seeking care only at MINSA (median 13.5 months, IQR 6.0-15.8) or at both NGOs and MINSA (median 23.0 months, IQR 14.0-70.0). Linkage to care at MINSA took a median of 3.5 months (IQR 3.0-4.3). Among those linked to care at MINSA who had received previous HIV care at NGOs, all had received study-based care at one or two NGOs, for a median of 2.0 months total (IQR 1.0-4.0).
Among the 6 PLHIV who were unstably linked to care, the time between diagnosis and data collection was a median of 38.5 months (IQR 18.5-105.0), with a median of 17.0 months with no HIV-related care (IQR 13.0-56.3). Four out of six PLHIV in this group reported prior HIV care at NGOs, with a median of 1.5 months of care (IQR 0.3-2.0). Among the 8 PLHIV with no post-diagnosis care, their diagnoses tended to be more recent, with a median of 12.0 months without HIV care (IQR 11.8-13.3).
One additional result is retention in HIV care following linkage and specifically, the number of changes in institution and/or care finance regimen. These changes occurred only among the 7 stably linked PLHIV who received care only at NGOs. In this group, 86% had a change in their HIV care institution or finance regimen (study-based versus free versus paid) following their first stable linkage, with one person reporting 4 changes.
Case studies while accompanying participants to MINSA establishments underscored the health system-related challenges in linking to care at MINSA: the two stages and multiple visits required to link to care after an HIV diagnosis, once the person has a confirmatory test result from the Peruvian National Institute of Health (INS) (Table 3). Stage 1 is a visit to the primary care establishment within whose jurisdiction the PLHIV lives, to secure a referral to a hospital that provides HIV care. Stage 2 corresponds to the hospital visits that the PLHIV is required to make to link to HIV care. During visits 2 and 3, the PLHIV receives a “routing card” and schedules the visits he needs to complete in order to link to care. From visits 4 forward, the PLHIV must complete each visit to obtain the necessary stamp/signature on the “routing card.” The PLHIV is not “approved” to link to HIV care until successfully completing this card. As shown in table 3, the accompanied linkage process took 12 visits over 80 days (2.7 months) for Case 1 and 12 visits over 114 days (3.8 months) for Case 2. For Case 1, the PLHIV spent 20.9 hours at health establishments during this period, including 3.5 hours with a service provider and 17.4 hours of administrative tasks and waiting time. For Case 2, it took 21.4 hours, with 3.2 hours of care and 18.2 hours of administrative tasks and waiting.
Figure 2 depicts the HIV care linkage and retention trajectories of 7 of the 34 PLHIV included in table 2. We collected this information from all 34 participants and present the 7 that best represent each sub-group (stably linked vs. unstably linked or unlinked; care at NGOs only vs. MINSA only vs. both NGOs and MINSA). The number of examples per sub-group is based on how diverse the trajectories were within each sub-group. Stably linked PLHIV who sought care only at NGOs (participants 1-3) were immediately enrolled in initial stable HIV care according to the protocol for study-based care or the evaluation of a health provider for non-study care. Following this initial stable linkage, this group had to change care institution and/or finance regimen – often doing so multiple times – in order to stay in care. They only achieved “truly” stable care upon agreeing to pay for care. Participant 1 had 11 months of study-based care followed by 5 months of free care before entering paid care, all at the same NGO. Participant 2 had 1 year 7 months of study-based care at one NGO before moving between 6 months of paid care at two NGOs and finally returning to the first NGO to continue paid care. Participant 3 had 10 months of no HIV care followed by 1 year 2 months of study-based care followed by three unsuccessful attempts to qualify for other studies at the same NGO and an additional 2 years and 10 months with no care. He later achieved stable care by linking to paid care at a second NGO. For stably linked PLHIV who sought care only at MINSA (participant 4), they took longer to link to initial stable care and then had no changes in care institution post-linkage. Participant 4 started linking very soon after diagnosis and took 4 months to complete a linkage process similar to the one shown in table 3. For stably linked PLHIV who sought care at both NGOs and MINSA (participant 5), they had repeat contacts with and repeat brief care at NGOs before linking to MINSA. Once linked to MINSA, they stayed in care there. Participant 5 received care at both NGOs and MINSA during the linkage process. He had two 2-month participations in research studies and four periods without care that totaled 5 years. After completing the linkage process in MINSA, both participants 4 and 5 – like the members of their respective sub-groups – were stably linked to care with no changes in institution or lapses in care post-linkage.
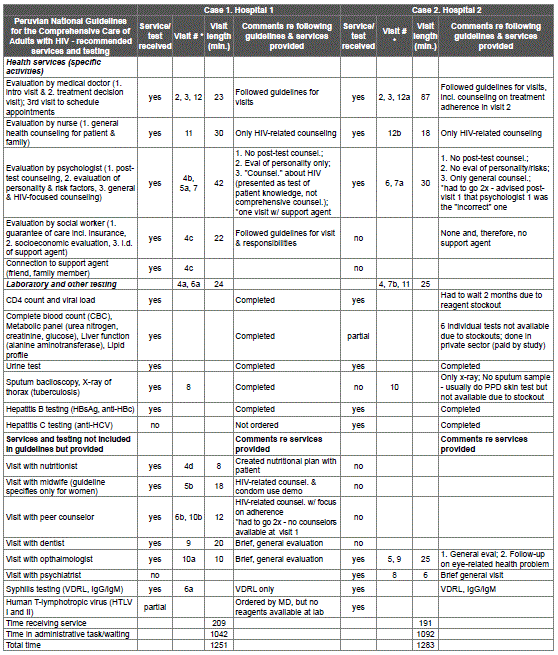
Table 3: HIV care linkage services recommended in national guidelines versus services received during observations of process at 2 hospitals, Ministry of Health, Lima, Peru, 2014-15
*Visit #s are whole numbers (1, 2) when only one service was provided at visit and numbers with letters (1a, 1b) when multiple services were provided at visit.
Among the 6 PLHIV who were unstably linked to HIV care postdiagnosis, 4 knew their diagnosis prior to baseline. Of these 4, 2 received care immediately following diagnosis, as follows: one paid for two continuous months of care, one month at two different NGOs, stopped care due to inability to pay, and remained without any care for 1.5 years at endpoint; and one received two months of care through an NGO study, stopped since he no longer wanted care, and remained without care 10 years later (not shown in Figure). The other 2 had more complex trajectories, both of which included long periods of linkage to stable HIV care, as shown in figure 2 (participants 6-7). Participant 6 linked to care through an NGO study immediately post-diagnosis and remained linked for 3 years 7 months until the study ended. After 8 months without care, he started linking to MINSA services through our study but then stopped due to a TB diagnosis and the time burden of daily directly-observed treatment (DOTS). Participant 7 alternated between stable HIV care (5 years in 2 prisons and 1 year 8 months in MINSA) and no HIV care (4 years 5 months). He then became unstably linked when he was referred from the MINSA health center where he was receiving pre-ART care (an establishment that does not provide ART) to a MINSA Hospital when he needed to start ART. He remained unstably linked 1 year 3 months later.
Finally, for some PLHIV, change in care institution or finance regimen resulted in certain practices related to ART. Some experienced changes in their ART regimens since different institutions administer different drugs and different study protocols specify different drug regimens. Others experienced one-time or repeated interruptions of their ART when facing challenges linking to and staying in care (see figure 2, participants 3, 5 and 6). Others alternated between taking ART and returning to pre-ART care, usually based on study protocol specifications (see figure 2, participant 5). Many of these changes were not based on review of the PLHIV’s medical record or on ART treatment failure or mal adherence, as good clinical practice indicates.
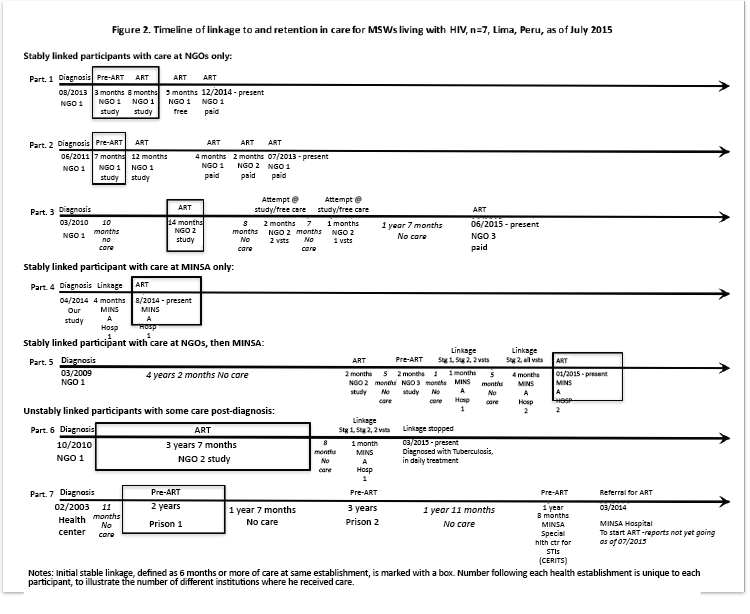
Figure 2: Timeline of linkage to and retention in care for MSWs living with HIV, n=7, Lima, Peru, as of July 2015
Our results from an innovative mix of close- and open-ended methods demonstrate that there are substantial drop-offs in the HIV care continuum for people at high risk of HIV and for PLHIV and substantial barriers linking to and remaining in HIV care, resulting in significant delays in linkage and high proportions of highly vulnerable PLHIV that remain unlinked or become unstably linked following initial stable linkage. Only 71% of MSWs reported a lifetime HIV test and only 56% received at least one lifetime confirmatory HIV test result. Among MSWs diagnosed with HIV, 76% had received at least one HIV care visit, but only 59% were currently in care, only 50% were currently on ART and an even lower 32% had been on ART for 6 months or more. One of the primary structural reasons for non-linkage to HIV care is the significant number of visits over several months needed to link to care in the Ministry of Health. Among MSW participants living with HIV who were stably linked to HIV care, the median time from diagnosis to stable linkage ranged from immediately to almost 2 years. Those who were unstably linked or unlinked reported a median of 1 to 1.5 years without any HIV care. Detailed linkage trajectories showed that the linkage process was very complex for many PLHIV and, surprisingly, that remaining in care at the same institution or under the same finance regimen post-linkage can be challenging, particularly at NGOs.
This study has strengths and limitations. First, we observed and documented the process of linkage to HIV care while accompanying MSW study participants, accompaniment that included close follow-up, motivation and economic support for miscellaneous costs. Given that these accompanied observations may provide a more positive view of the linkage process, we surveyed a larger group of participants about their experiences with the HIV care continuum post-diagnosis, in order to document the linkage process with little to no intervention. However, the reality for our participants is that only 6 of 34 were linked prior to our study. We provided lesser intervention (education and orientation to link) for most unlinked participants and greater intervention (accompaniment to link) for fewer unlinked participants. Therefore, results here may present a more positive image of linkage to HIV care in this population than what may have taken place without our intervention. Second, our study focuses on factors related to the health system and does not explore individual or personal barriers and facilitators such as HIV-related stigma and coping [35]. Third, participant reports of their engagement in the HIV care continuum may be subject to recall and social desirability bias. We strove to minimize both by using an open-ended format to literally walk participants through their post-diagnosis timeline in a nonintimidating manner. Finally, we do not have data on viral suppression, a key component of the HIV care continuum.
Our results are among the first in Peru to document the HIV care continuum in a large population of MSM. This study is the first we are aware of to ask participants about their engagement in the care continuum, in comparison to national data and past studies that compiled data from different sources to estimate the number of individuals engaged in different points in the continuum. Peru’s national data is limited to the estimated number of people living with HIV (72,000) and the actual number currently taking ART (21,479). One study based on sub-group data estimated that among the 38,000 MSM and TW living with HIV, 27% knew their status, 25% had accessed HIV care, 24% were on ART, and 18% had an undetectable viral load [15]. This actually represents very high access to HIV care since it translates into, among MSM and TW who know their status, 94% accessing HIV care and 89% being on ART. Compared to the study with MSM/TW just cited, our study found that: a much higher proportion of all MSWs (56%) were aware of their HIV status, but a much lower proportion of our MSWs who knew their HIV status were linked to HIV care (59%) and an even lower proportion were on ART (50%). At endpoint, even with significant support from our study to link to and stay in care, 59% of PLHIV participants were currently linked to HIV care. Further work is needed to describe engagement in the HIV care continuum among overall and sub-groups of Peruvians.
Another important result is the description of the HIV care continuum in Peru, specifically the distance between the steps. Globally, the HIV care continuum has been characterized as fluid, with seemingly equidistant steps [12]. However, in Peru there is an initial gap between the application of the HIV screening test and the receipt of the HIV test result. Then, there is an even larger gap between an HIV diagnosis and linkage to pre-ART care and, if needed, ART. Studies in sub-Saharan Africa found similar drop-offs in the continuum during the multiple visits that comprise preART care [36-39]. We focused on documenting objective dimensions of the distance between steps in the continuum, i.e. the visits and time needed to link to care. However, there are other dimensions that warrant consideration, including time, costs and mental health. PLHIV in Peru need to return for about 10 originally scheduled visits and for additional visits due to scheduling errors and shortages of reagents for CD4 T-cell count and viral load testing that can last several months, sometimes with lengthy waiting periods between visits. This is much more complex and time-consuming than the processes documented in a recent review of 28 studies on retention in pre-ART care in Africa, which found that linkage to pre-ART care takes 1-2 visits over a period of 2 days to 2 weeks [38]. PLHIV in Peru also need to pay for multiple transport and other costs such as food during lengthy visits and tests in the private sector to avoid lengthy waits due to stockouts. Another key dimension is mental health. Vulnerable populations, as with our study population, have a higher likelihood of contracting HIV and an HIV diagnosis brings additional vulnerabilities [40]. Presenting PLHIV who have layered vulnerabilities with the challenge of linkage to HIV care may negatively influence their mental health if the process – as in the case of Peru – is complex and timeconsuming. This process may also discourage PLHIV from continuing the linkage process.
Our findings also show that an unintended result of the multidisciplinary care provided by the Peruvian government is the burden for PLHIV of navigating HIV care options and the dynamics and requirements of securing care. The Peruvian National Guidelines for the Comprehensive Care of Adults with HIV were formulated to ensure high-quality, comprehensive care for PLHIV [41]. However, an unintended result of these multiple components– and particularly, of required completion of these components to enter HIV care – is the significant burden on PLHIV. At a recent national expert meeting on HIV in Peru, participants discussed one of the motives behind requiring that PLHIV complete the multidisciplinary care visits prior to entering HIV care: the belief of health system leadership and personnel that individuals who are able to persist in completing the MINSA “routing card” demonstrate that they are committed to receiving HIV care and are more likely to be adherent to ART [42]. However, this approach runs counter to the right of all PLHIV to access HIV care and treatment [43]. It also runs counter to recent evidence on the benefits of early ART for PLHIV, related population groups, and health and other social welfare systems [1,2,5,31] and to the recently-modified WHO recommendations to initiate ART regardless of the CD4+ T cell count [18].
An additional, surprising finding is that engagement in HIV care can be unstable when PLHIV have already linked to the health care system, particularly NGOs, or when they have been in pre-ART care in MINSA and need to transition to ART at another MINSA establishment. If PLHIV were in HIV care at an NGO, the initial stable linkage to care was usually significantly faster and easier than at MINSA. However, some PLHIV were unable to link to study-based or free NGO care quickly and easily and, if unable or unwilling to pay for care or seek care at MINSA, often spent significant periods without any HIV care. Continued engagement in HIV care at NGOs was also a challenge, with individuals needing to move from study to study, negotiate and self-advocate to secure free care, or pay for care. The health system needs to better coordinate HIV care options, including improved integration and referral systems across NGOs and MINSA and across MINSA establishments, and provide support for PLHIV already engaged in care. Alternative, decentralized models of HIV treatment delivery such as community adherence clubs and community distribution sites could be considered to lower patient barriers [44,45]. The system also needs to better integrate HIV and TB care. Although this integration exists in Peru, if the PLHIV has not yet achieved stable linkage to HIV care when diagnosed with TB, the burden continues to fall on him to complete the linkage process for HIV care, while also completing daily TB treatment.
Another important finding is regarding interruptions in ART, which have important negative individual and population consequences including increased viral load, declining immune status with clinical progression [46], and increased likelihood of HIV transmission and development of ART resistance [2]. Studies have also identified resistance to ART that develops after interruptions in ART [47-50]. This result further affirms the need to coordinate care across HIV care providers.
Further research is needed to characterize engagement in the HIV care continuum among other sub-groups of Peruvians and structural and individual barriers and facilitators of this engagement. Urgent implementation science research is needed to expand access to HIV testing and results of that testing, facilitate linkage to HIV care for PLHIV, and develop strategies to better integrate HIV care options to aid the linkage process and strengthen retention in HIV care post-linkage. Even if Peru moves toward the recent WHO guidelines of immediate initiation of ART, the structural issues highlighted in this paper will be critical to address in order to better support PLHIV to move quickly into and remain in HIV care.
The authors would like to thank the study participants who shared their experiences during this study. This work was supported by NIH Fogarty International Center grant K01TW009206 and National Institute of Mental Health grant R21MH098982.
The authors declare that they have no competing interests with respect to the research, authorship or publication of this article.
Download Provisional PDF Here
Article Type: Research Article
Citation: Bayer AM, Díaz CM, Chiappe M, Baker AN, Egoavil MS, et al. (2016) The Odyssey of Linking to and Staying in HIV Care among Male Sex Workers in Peru. J HIV AIDS 3(1): doi: http://dx.doi.org/10.16966/2380-5536.134
Copyright: © 2016 Bayer AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access