Introduction
Neuroinflammation is a pathogenic factor of neurological disorders,
such as HIV-associated dementia [1], Alzheimer’s disease [2], and
Parkinson’sdisease [3]. Such inflammation is usually a result of prolonged
activation of microglia and astrocytes, and the subsequent release of
pro-inflammatory cytokines and reactive oxidative species (ROS). Both
microglia and astrocytes can be infected by HIV and serve as reservoirs for
the virus [4]. During HIV and SIV infection, acute inflammatory response
in the central nervous system (CNS) was observed several days after the
infection [5], and severer neuroinflammation was found in patients with
HIV-associated neurocognitive disorders (HAND) than patients without
HAND [6]. In HIV-infected brain, the hippocampus hosts higher HIV
viral load than the cerebellar cortex and mid-frontal cortical gray matter
[7], expresses high levels of HIV chemokine co-receptors which facilitates
neuronal loss and gliosis [8], and suffers greater immunoreactive
neuronal loss compared to the frontal cortex [9]. The hippocampus is
also a major inflammation site in the brain with antiviral treatments [10],
as the inflammation (indicated by CD68 expression) did not seem to be
alleviated by HAART as seen in the basal ganglia [4].
NFκB is a pro-inflammatory transcription factor that regulates the
expression of more than 400 genes, and can be activated by many stimuli,
such as proinflammatory cytokines, virus and viral proteins [11]. Abnormal
NFκB activity is involved in the pathogeneses of chronic inflammation
and neurodegenerative diseases. NFκB consists five subunits: RelA (p65),
RelB, c-Rel, NFκB1 (p50/105) and NFκB2 (p52/p100), and the p50-p65
heterodimer is the most abundant functional NFκB complex [12].
AP-1 is another inducible pro-inflammatory transcription factor,
composed of the Fos family, Jun family and ATF family. c-Jun is the major
component of AP-1 and its basal expression is detected in many cell types
and compartments in the brain [13]. Increased c-Jun expression-induced
cell death in the CNS has been found in Alzheimer’s disease and cerebral
ischemia [14].
Inflammatory cytokines interleukin 1 beta (IL-1β) and tumor necrotic
factor alpha (TNFα) can be transactivated by NFκB and AP-1, and once
secreted, they further stimulate NFκB and AP-1 activation through their
receptors to form a positive feedback circle. Both astrocytes and microglia
can release IL-1β and TNFα [15], and increased IL-1β has been reported
in the brain of HIV patients [16]. Chronic release of these cytokines results
in neuronal damage through ROS generation and calcium influx, as well
as through increasing monocyte infiltration in the brain [17].
Varied extracts derived from bamboo plants have been used in
Traditional Chinese Medicine to treat diseases, including inflammation.
Phyllostachys edulis, also known as Maozhu or Moso, is one of the fastest
growing plants in the world. The leaves of P. edulis is a by-product of the
bamboo timber industry, and a patented procedure has been developed
in China to utilize this “industrial waste” to produce a bamboo extract
(BEX). In our previous studies, we have shown that BEX as a dietary
supplement decreased inflammation in the peripheral circulation, as well
as decreased anxiety in obese mice [18,19], and the anti-inflammatory
effect of BEX was partially mediated by inhibiting the activation of NFκB
and AP-1 [20].
HIV-1 transgenic (TG) rat is an animal model used in HIV-neuro
AIDS studies. These rats constitutively express 7 HIV viral proteins (vpr,
env, nef, vif, vpu, rev, and tat), and neuroinflammation, as evidenced by
upregulated IL-1β, TNFα, and NFκB, has been reported in homogenized
brain hemisphere [21]. In this study, we specifically examined the
inflammatory status in the hippocampus of the TG rats, and evaluated the
anti-inflammatory effect of BEX.
Materials and Methods
Bamboo extract (BEX)
BEX used in this study was provided by Golden Basin LLC (Honolulu,
HI). It was produced by Golden Basin Bio-Tech (Hunan, China) through a
patented procedure (Chinese invention patent, CN 1287848A). This BEX
is commercially available in the United States as a dietary supplement.
To produce BEX, twigs of Phyllostachys edulis no longer than 2 feet were
washed in water and air dried, ground and infused with 70-90% ethanol
twice. The ethanolic extract was concentrated by vacuuming. The final
product contains 46% moisture, and the dry mass contains 53 mg/g
polyphenols, 3 mg/g fat, 67 mg/g total sugar, and 233 mg/g protein.
Animal and dietary treatment
Ten (10) one-month-old HIV-1 NL4-3 gag/pol transgenic (TG) rats
and 5 genetic background control Fisher 344 (F344) rats were purchased
from Harlan Inc. (Indianapolis, IN) and housed at the Laboratory Animal
Service facility of the University of Hawaii. The rats were maintained
on a 12-hour light/dark schedule. Food and water were accessible ad
libitum. Body weight and food consumption were monitored weekly. The
experimental procedures were approved by the Institutional Animal Care
and Use Committee (IACUC) of the University of Hawaii.
After one week of acclimation, 5 F344 rats and 5 TG rats were fed a
standard (control) diet, and the other 5 TG rats were fed the standard
diet supplemented with BEX at a dose of 11 grams dry mass per 4057
Kcal, or 1% w/w. Both diets were purchased from Research Diets (New
Brunswick, NJ). The dietary composition has been reported in our
previous publication [18].
Sample preparation
The rats were euthanized in a CO2 induction chamber when they were
10-month old. The whole brain weight was measured and hippocampus
was dissected on ice and stored at -80°C. The hippocampal tissue was
then powderized on dry ice. An aliquot of the powder was sonicated in
PBS (except for samples prepared for western blot, as described below),
centrifuged at 18,000 × g for 10 min at 4°C, and the supernatant was
collected. The protein concentration of the supernatant was measured
using Bradford assay (BioRad, catalog No. 500-0205). The samples were
stored at -80°C until assayed.
Chemicals and instruments
All chemicals used in this study were purchased from Sigma (St.
Louis, MO) unless otherwise noted. A SpectraMax 340 from Molecular
Devices (Sunnyvale, CA) was used for HNE-His ELISA assay. A protein
electrophoresis system from BioRad (Hercules, CA), and an Odyssey
Infrared Imaging System and an Odyssey Application Software Version
3.0 (Li-Cor Biosciences, Lincoln, NE) were used in western blot. A Light
cycler 480 II (Roche Applied Science, Indianapolis, IN) was used in Realtime
PCR.
Western blot
Hippocampal tissue powder was sonicated in 1M Tris (pH 7.5)
membrane lysis buffer containing 1M NaCl, 1% Trition X-100, 5 mM
EDTA, proteinase inhibitor, and phosphatase inhibitor. Supernatant
was collected after 10 min centrifugation at 18,000 × g, 4°C. Protein
concentration was measured by Bradford assay. Primary antibodies goat
anti-Iba1 (sc-28528), rabbit anti-c-Jun (sc-1694) and rabbit anti-IL-1β
(sc-7884) were purchased from Santa Cruz (Dallas, TX), rabbit antiGFAP
(ab7260) and rabbit anti-NFκB p65 (ab7970) were purchased from
Abcam (Cambridge, MA); Secondary antibodies were purchased from LiCor
(Lincoln, NE). Other western blot procedures have been reported in
details in our previous publication [22].
Quantitative real-time PCR
Total RNA was extracted from hippocampus using Trizol (Invitrogin,
Grand Island, NY) and cleaned up using RNeasy mini kit (Qiagen,
Valencia, CA). The reverse transcription kit for cDNA synthesis was from
Applied Biosystems (Foster City, CA). SABiosciences SYBR® Green (PA-
010-24) kits were used for quantitative PCR. Sequences of the following
primers were obtained from the Universal Probe Library of Roche Applied
Science and synthesized by Integrated DNA Technologies (Coralville, IA):
β-actin (actin) forward: cccgcgagtacaaccttct, reverse: cgtcatccatggcgaact;
GFAP forward: tttctccaacctccagatcc, reverse: gaggtggccttctgacacag;
ionized calcium-binding adapter molecule 1 (Iba1) forward:
ccgaggagacgttcagttactc, reverse: tggcttctggtgttctttgtt; interleukin 1 beta
(IL1β) forward: tgtgatgaaagacggcacac, reverse: cttcttctttgggtattgtttgg;
tumor necrosis factor α (TNFα) forward: tgaacttcggggtgatcg, reverse:
gggcttgtcactcgagtttt.The reactions were carried out in quadruplicates.
Statistical analysis
Prism 5 (GraphPad Software Inc., La Jolla, CA) was used for statistical
analysis. Differences among the means were analyzed using one-way
ANOVA and Bonferroni’s multiple comparison test in figure 1, Mann
Whitney test, Kruskal Wallis test, and Dunn’s post-hoc test in figures 2-4.
Correlation in figure 2 was analyzed using linear regression. p<0.05 was
considered statistically significant.
Results
Energy consumption, body and brain weight
The energy consumption and body weight were recorded weekly for 30
weeks. No difference of energy intake was observed among the 3 groups
when the weekly records were averaged (Figure 1A). At the end of the
study (when the rats were 42-week-old), the average body weights of the
3 groups were different (p=0.0053, one-way ANOVA, Figure 1B), i.e. TG
and TG+BEX rats were significantly lighter than the F344 rats (-12.6%,
TG vs. F344, -12.4%, TG+BEX vs. F344, p<0.05, Bonferroni’s post-hoc).
Neither wet brain weight nor the ratio of brain weight over body weight
showed differences among the 3 groups (Figures 1C and D).
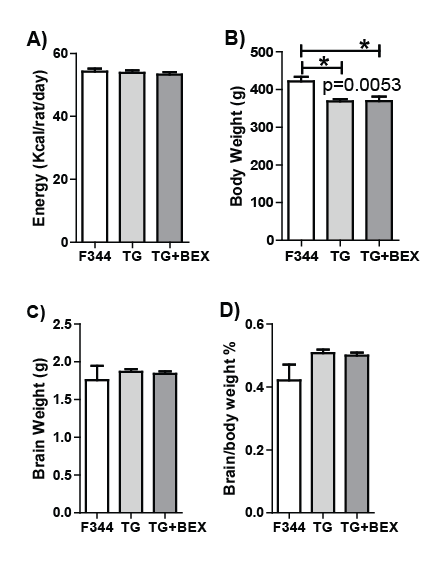
Figure 1: Energy consumption, body and brain weight of F344 rats fed control diet (F344), HIV-1 transgenic rats fed control diet (TG), and HIV-1
transgenic rats supplemented with BEX (TG+BEX). A: Energy consumption over 9 months. B: Bodyweight before decapitation. C: Wet brain weight.
D: Percentage of brain weight over body weight. Average and SD are shown, n=5 per group. The P value labeled in panel B was from one-way
ANOVA.*p<0.05, Bonferroni’s multiple comparison
HIV-1 transgenesis-induced glial activation and itsattenuation
by BEX
To study HIV-1 transgenesis-induced inflammation in the
hippocampus, the expression of astrocyte marker (GFAP) and microglia
marker (Iba1) were measured. TG rats fed control diet showed almost 7
folds increase of GFAP protein level compared to F344 rats (Figures 2A
and 2B, p=0.0079, Kruskal-Wallis test). This increment was significantly
inhibited by BEX supplement (p<0.05, Dunn’s post hoc test), and as a
result, the protein levels of GFAP in the F344 rats and TG+BEX rats were
similar. Conversely, the mRNA levels of GFAP did not show difference
among the 3 groups (Figure 2E).

Figure 2: Protein and gene expression of glial fibrillary acidic protein (GFAP) and ionized calcium-binding adapter molecule 1 (Iba1)in the hippocampus
of F344 rats fed control diet (F344), HIV-1 transgenic rats fed control diet (TG), and HIV-1 TG rats supplemented with BEX (TG+BEX). A: Western
blot image of GFAP, Iba1, and loading control β-actin. B: Relative quantification of GFAP protein expression. C: Relative quantification of Iba1 protein
expression. D: Correlation between the protein levels of GFAP and Iba1. E: Relative mRNA expression of GFAP.F, Relative mRNA expression of Iba1.
Average and SD are shown, n=5 per group. P values labeled in panels B and C were from Kruskal-Wallis test; and that in panel D was from linear
regression. #p<0.05, Dunn’s multiple comparison test; *p<0.05, Mann Whitney test. For western blot, all samples were run on the same gel.
The protein expression of Iba1 was significantly decreased in the TG
rats fed control diet compared to that in the F344 rats (-92.5%, p=0.003),
but BEX supplement in the TG rats increased Iba1 protein by almost 40
folds (p=0.016), as shown in Figures 2A and 2C. Interestingly, the protein
expression of GFAP and Iba1 showed strong negative correlation when
data from all samples were pooled (Figure 2D, r=-0.92, p<0.0001). No
difference of the Iba1 mRNA expression was found among the 3 groups
(Figure 2F).
HIV-1 transgenesis-induced upregulation of cytokines and its
reduction and normalization by BEX
As shown in Figures 3A and 3B, the protein level of IL-1β in the
TG rats fed control diet was 1.4 folds higher than that in the F344 rats
(p<0.05, Dunn’s post-hoc), and this increment was normalized by BEX
supplement, as indicated by a 37% decrease of IL-1β expression in the
TG+BEX rats compared to the TG rats fed control diet (p=0.056, MannWhitney
test). The IL-1β levels in the F344 and TG+BEX groups were
comparable. Similar changes were also observed on the mRNA level of
IL-1β (Figure 3C), i.e., the highest IL-1β mRNA level was found in the TG
rats fed control diet, which was 90% higher than the F344 group (p=0.016,
Mann-Whitney test) and 170% higher than the TG+BEX group (p<0.01,
Dunn’s post-hoc). The TG+BEX rats also showed lower IL-1β mRNA level
than the F344 rats (-38.4%, p=0.03, Mann Whitney test). When mRNA
expression of TNFα was tested (Figure 3D), higher TNFα mRNA level
was found in the TG rats fed control diet compared to the TG+BEX rats
(+113%, p=0.03, MannWhitney’s test, Figure 3D).

Figure 3: Protein and gene expression of interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα) in the hippocampus of F344 rat fed control diet
(F344), HIV-1 transgenic rats fed control diet (TG), and HIV-1 transgenic rats supplemented with BEX (TG+BEX). A: Western blot image of IL-1β
and loading control β-actin. B: Relative quantification of IL-1β protein expression. C: Relative quantification of IL-1β mRNA expression. D: Relative
quantification of TNFα mRNA expression. Average and SD are shown, n=5 per group. P values in panels B and C were from Kruskal-Wallis test.
#p<0.05, Dunn’s multiple comparison test; *p<0.05, Mann Whitney test; ^p=0.056, Mann Whitney test. For western blot, all samples were run on the
same gel.
HIV-1 transgenesis-induced upregulation of transcription
factors and its normalization by BEX
To understand the transcriptional regulation of the cytokines, the
protein expression of p65 (a subunit of NFκB) and c-Jun (a subunit of
AP-1) were measured (Figure 4). The p65 protein expression level was
different among the three groups (p=0.038, Kruskal Wallis test), and it
was significantly lower in the TG+BEX rats compared with the TG rats
fed control diet (-42.6%, p<0.05, Dunn’s post-hoc, Figure 4B). The protein
expression of c-Jun was also different among the three groups (p=0.02,
Kruskal Wallis test, Figure 4C), with significantly higher c-Jun expression
in the TG rats fed control diet than the F344 rats (+40.4%, p=0.016,
Mann-Whitney test) and the TG+BEX rats (+113%, p<0.05, Dunn’s posthoc).
While the F344 and TG+BEX groups showed similar protein levels
for both p65 and c-Jun.

Figure 4: Protein expression of p65 and c-Jun in the hippocampus of F344 rat fed control diet (F344), HIV-1 transgenic rats fed control diet (TG), and
HIV-1 transgenic rats supplemented with BEX (TG+BEX). A: Western blot image of p65, c-Jun and loading control β-actin. B: Relative quantification
of p65 protein expression. C: Relative quantification of c-Jun protein expression. Average and SD are shown, n=5 per group. P values in panels B
and C were from Kruskal-Wallis test. #p<0.05, Dunn’s multiple comparison test; *p<0.05, Mann Whitney test. For western blot, all samples were run
on the same gel.
Discussion
Astrogliosis has been reported in both HIV-infected patients [4] and
animal models [23,24]. We showed increased GFAP protein expression in
the hippocampus of the TG rats, which is consistent with the hippocampal
inflammation observed in HIV patients [4]. However, using the same
animal model, Rao et al. [21] reported no changes on mRNA and protein
levels of GFAP in the left hemisphere of the TG rats. This difference may
be due to the following reasons: (1) age difference, the rats in the study
of Rao et al. [21] were 1-3 months younger than those used in our study;
(2) Rao et al. [21] used the cytosolic fraction for western blot, while
we extracted proteins using a membrane lysis buffer, which could have
released compartmentalized proteins; and (3) Rao et al. [21] studied the
combined effect in multiple brain regions, while we focused on a defined
region. Rao et al. [21] reported increased mRNA and protein levels of IL-
1β, TNFα and protein level of NF-κB subunit p50, which were inline with
our observations. A different research group also used this animal model
for inflammation study, and they reported upregulated protein levels
of TNFα, IL-1β, and GFAP in the frontal cortex and subcortical white
matter, implicating neuroinflammation in other brain regions besides the
hippocampus [24].
As a commonly used microglial activation marker, Iba1 expression has
been found increased in the CNS of patients with HIV encephalitis [25],
as well as in the spinal cord [26] and caudate-putamens [27] of rats treated
with gp120. In 4-to-5-month-old HIV-1 TG rats, increased abundance
of Iba1 positive microglial cells were found in both hippocampus and
neocortex, and the change was more prominent in the hippocampus
compared to the neocortex; these cells also displayed abundant branches
and processes and distended cytoplasm, suggesting the possibility of an
activated state [28]. However, our study showed decreased Iba1 expression
in the hippocampus of the TG rats. In line with our finding, Rao et al. [21]
also reported that in the hippocampus of 7-month old HIV-1 TG rats,
the Iba1-positive microglia showed decreased arbor complexity and ~50%
shortened processes compared to control [21]. Therefore the decrease
of hippocampal Iba1 expression found in our study may be associated
with the morphology changes of the Iba-positive microglia in the HIV-
1 TG rats. Furthermore, a study of Cerbai et al. [29] showed that the
number of Iba1-positive reactive microglia significantly decreased in
the CA1 Stratum radiatum of the hippocampus of aged (22-month)
rats compared to adult (3-month) rats, while the number of resting
microglia remained the same [29], implicating that microglial activation
is age-dependent. The HIV-1 TG rats in our study were 10 months,
and potential premature aging in these rats may have at least partially
caused the decrease of Iba1 in the hippocampus. Interestingly, Cerbai
et al. [29] also showed spatial reciprocal interaction of microglia and
astrocytes around apoptotic neurons [29], which might be a potential
explanation for the inverse correlation between the protein levels of
GFAP and Iba1 found in our study.
Our previous studies showed that BEX inhibited NFκB and AP-1
activation under lipotoxic conditions [20], and prevented obesityinduced
inflammation in peripheral circulation [19]. Bioactivity-guided
fractionation revealed that flavonoids such as tricin and 7-O-methyltricin
were among the anti-inflammatory compounds in BEX [30]. In the
present study, BEX inhibited the increases of both mRNA and protein
levels of IL1β in the hippocampus of the HIV-1 TG rats, and meanwhile
lowered the protein levels of p65 and c-Jun, implicating the inhibition of
both NFκB and AP-1 pathways. PPARγ upregulation has been reported
to attenuate NFκB and AP-1 signaling [31], and interestingly our
unpublished in vitro data suggested that BEX was able to enhance the gene
expression of PPARγ. NFκB activation is also linked to the upregulation of
GFAP [32], which provides an explanation to the GFAP over expression
in the hippocampus of the HIV-1 TG rats, and the protective effect of
BEX. Lastly, NF-κB is needed for HIV viral gene transcriptional activation
through the binding of p50/p65 and c-Jun at the long terminal repeat
(LTR) [33], whether BEX can reduce HIV replication through inhibiting
NF-κB activity is to be further studied.
It is arguable that since BEX inhibited multiple protein expressions
in the hippocampus of the TG rats, it is possible that BEX might have
caused hippocampal atrophy. To exclude this possibility, we also evaluated
the spatial learning ability (which is closely related to hippocampal
function) of the rats 2 months before the endpoint using a modified
Morris water maze [34]. We found that after 2 weeks of training, it took
the TG rats 2.4 folds longer time to find the hidden platform than the
F344 rats did, and BEX supplement shortened this latency in the TG rats
by 36% (Supplemental Figure 1). This result showed that BEX supplement
seemingly improved the hippocampal function, and therefore should not
have caused hippocampal atrophy.
In conclusion, this study demonstrated neuroinflammation in the
hippocampus of the HIV-1 TG rats, as evidenced by higher expression
levels of GFAP and IL1β, and this inflammatory status was effectively
abolished by dietary supplement of BEX through inhibiting the NF-κB
and AP-1 signaling.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Author’s Contributions
XP carried out experiments, analyzed and interpreted data, and drafted
and revised the manuscript. JP designed the study, interpreted data and
critically revised the manuscript.
Acknowledgements
This study was made possible by NIH grants R21 AT005139,
R21AT003874, G12MD007601 (RCMI/ BRIDGES) and R24 PAR09-011
(DIDARP). Its contents are solely the responsibility of the authors and do
not necessarily represent the official views of the NIH.





