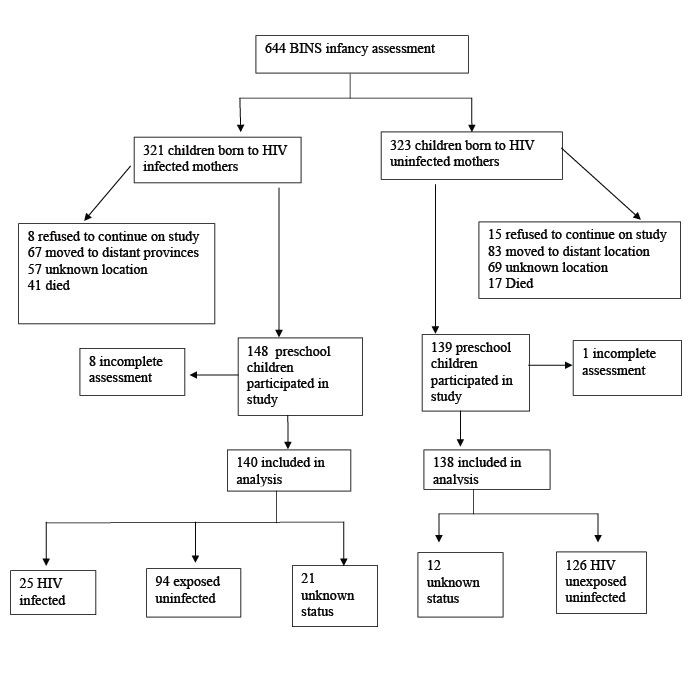
Figure 1: Flow chart showing the children followed up at preschool age

Kandawasvika GQ1 Mapingure PM2 Pazvakavambwa IE1 Stray-Pedersen B3
1Department of Paediatrics and Child Health, University of Zimbabwe College of Health Sciences, Zimbabwe*Corresponding author: Dr Gwendoline Q. Kandawasvika, Department of Paediatrics and Child Health, University of Zimbabwe College of Health Sciences, P.O. Box A 168 Avondale, Harare, Zimbabwe, Tel: 00263772235643; E-mail: gwenkandawasvika@gmail.com
Background and purpose: The effect of paediatric HIV infection on neurodevelopment at preschool age is under reported in Sub -Sahara Africa where the burden lies. This study assessed cognitive outcome and health status at preschool age among Zimbabwean children, enrolled from a national PMTCT program, with vertically transmitted HIV type 1 infection followed up for 5 years.
Methods: Cognitive function was assessed in 278 preschool children with Kaufman short form of McCarthy Scales of Children`s Abilities (MSCA): 25 HIV infected, 94 HIV exposed uninfected, 126 HIV unexposed uninfected (controls) and 33 with unknown HIV status. Only115 had received prophylactic single dose nevirapine at delivery, but none were on antiretroviral treatment.
Results: HIV infected children did not differ significantly in global cognitive ability compared to their age matched HIV exposed uninfected and HIV unexposed uninfected peers of similar background. Mean scores were significantly lower between HIV exposed uninfected and HIV unexposed uninfected children in specific subtests representative of verbal, perceptual performance and quantitative performance. HIV infected children were significantly more stunted, had more skin disorders, and lymphadenopathy compared to their uninfected peers. Maternal age and family income were predictive of lower GCI in this study.
Conclusion: HIV infected children who survive to preschool age did not manifest lower cognitive scores than established child norms. The prevalence of cognitive impairment in this study was 8.6% and was more frequent in HIV uninfected children. Chronic malnutrition as indexed by stunting was associated with poor cognitive function at preschool age. Therefore comprehensive interventions to prevent childhood HIV and malnutrition could result in significant improvement in cognitive function at preschool age and later academic achievement.
HIV; Neurocognitive Consequences; Preschool children
As the world embraces the new Sustainable Developmental Goals, focus should now project beyond improvement in child survival, but improving the development indicators for those children who survive. The period from conception to the first six years of life has been shown to set a basis for the coping skills in learning, behavior and health in adulthood [1]. In developing countries it was estimated that more than 200 million children under the age of 5 years do not reach their cognitive developmental potential due to preventable risk factors including malnutrition, inadequate cognitive stimulation, infections such as HIV [2]. Children living in resource-constrained communities suffer the greater burden of these risk factors. These early adverse childhood experiences influences brain development and have negative social, economic and political ramifications for the future [3].
The earliest known case of infection with HIV-1 in a human was detected in a blood sample collected in 1959 from a man in Kinshasa, Democratic Republic of Congo [4]. Since then, globally by 2011, an estimated 21.7 million people had died due to HIV infection whilst another 34 million were living with the infection [5]. Of the 3.4 million children living with HIV infection worldwide, 91% live in sub-Sahara Africa and the majority acquired the infection through vertical transmission [6]. HIV type 1 is associated with increased risk for central nervous system disease in children as the virus easily penetrates the immature blood brain barrier. CNS invasion occurs during primary infection and is often followed by compartmentalization. HIV invades cells of the lymphatic system in the nervous system by binding to the CXCR4 and CCR5 chemokine receptors, respectively. Infected monocytes and T lymphocytes pass from the lymphatic system to the CNS in order to invade the nervous system [7]. The infected immune cells release numerous chemokines that either damage or kill neurons directly or stimulate other un-infected cells to produce inflammatory and neurotoxic factors. Both of these mechanisms lead to neuronal injury or death via excitatoxicity, oxidative damage, and apoptotic pathways [8]. In perinataly infected children cognitive, motor and behavioral disorders have been shown to be related to the direct infection of the CNS by the HIV virus as the disease progresses [9]. The postnatal period of brain development is particularly susceptible to excitatory neuronal damage due to the active synaptogenesis and pruning that takes place at this age [10].
The relationship between paediatric HIV infection and neurodevelopment has been studied mainly in developed countries [11] and yet the greatest burden of paediatric HIV is in sub-Sahara Africa. Studies conducted in developed countries focused on children receiving antiretroviral treatment and whose mothers were on recreational drugs, which is a different contextual setting from sub Saharan Africa where antiretroviral therapy was not universally available to all children [12]. Blanchette and Fishkin investigated American preschool and school age children respectively and reported no gross cognitive differences between infected and uninfected peers from similar backgrounds [13,14]. However, a study among Ugandan children reported global deficits in HIV infected children compared to HIV uninfected [15], findings corroborated by evidence from the DRC among Zairian preschool children [16].
In resource constrained settings, one of the reasons for lack of information on child disability is the unavailability of experts and culturally sensitive neurodevelopmental assessment tools. The comparison of research findings on the effect of perinatal HIV infection on child neurodevelopment is further hampered by the lack of standardized validated tools. A few culturally appropriate assessment tools have been developed for different African cultures [17,18]. Although comparison between groups is possible, these tools were created for children of a specific area of residence (rural or urban) and for a limited age range, which limits interpretation of outcome measures when employed in a different setting to that of origin.
There is a dearth of knowledge on the relationship between perinatally acquired HIV infection and cognitive abilities at preschool age in African children. Of note is the sad reality that interventions aimed at preventing vertical transmission of HIV or the early infant diagnosis and treatment are not readily available [19]. Inevitably, the numbers of children infected or exposed to HIV are expected to increase, yet there is lack of information on the neurodevelopmental performance of children as they mature from infancy to adolescence. Disability among children was estimated at 2% in Zimbabwe in 2000 [20], but no documented evidence exist on the contribution of HIV-1 at preschool age. The aim of this study was to describe cognitive at preschool age and identify predictors of cognitive function, in children born to HIV infected and uninfected mothers who participated in the national PMTCT program.
The study was part of the Better Health for the African Mother and Child cohort study (BHAMC) whose aim was to explore the role of sexually transmitted infections in pregnancy outcomes, details have been presented elsewhere [21,22]. Briefly from 2002 to 2004, a total of 1050 pregnant women at 36 weeks of gestation with a documented HIV results were enrolled from a national PMTCT programme in Zimbabwe: 479 (46%) HIV infected women and 571 (54%) uninfected. During the study period, a total of 17528 pregnant women delivered at the study sites whilst the national the HIV prevalence rate among pregnant women attending ante natal clinics was estimated at 23% [23]. From the initial cohort of 644 children, twenty three declined to participate for various reasons, 150 had relocated to a distant region, 58 had died, and 126 could not be traced, resulting in 287 who agreed to participate (Figure 1). A total of 278 preschool children had complete examinations and were included in the analysis. Comparison of maternal characteristics at baseline between the study participants and those who did not participate at preschool age showed no significant difference in relation to marital status, employment, years spent in formal education and occupation. Mothers lost to follow up were more likely to be younger and less number of living children.

Figure 1: Flow chart showing the children followed up at preschool age
The women `s baseline socioeconomic demographics were collected and in the case of mothers infected with HIV, information on the use of prophylactic intrapartum single dose nevirapine in the mother–infant pair was documented, as was the standard practice as the time of the study. At the time CD4 counts were not routinely available for HIV infected women. The women were reviewed at scheduled visits at 6 weeks, 4 months, and 9 months and 15 months where screening for reproductive tract infections and in the case of HIV uninfected women, blood was drawn for HIV screening. Neither the HIV unexposed uninfected mothers who participated in neither the neurodevelopmental assessments nor their infants, sero-converted for HIV infection during the study period. Their infants were not tested further for HIV infection. The infants were reviewed at 6 weeks, 3 months, and every 3 months until the age of 24 months, then yearly thereafter for growth, development assessments and clinical care. HIV exposed infants` HIV infection status were determined by HIV DNA PCR (Roche Diagnostics Indianapolis, USA) if the children were aged less than 15 months and rapid HIV antibody tests Determine (Abbot Diagnostics, Indianapolis, USA) and Oraquick (Abbot Diagnostics, Indianapolis, USA) at age 15 months. The proportion of HIV infected infants has been reported previously [24].
The study was approved by the Medical research council of Zimbabwe and the Norwegian ethical review committee.
This was a sub study in a prospective cohort of pregnant women, infected and uninfected with HIV, enrolled at 36 weeks of gestation, whose children were followed up from birth to 10 years [25]. Neurodevelopmental assessments were conducted cross sectional in infancy and in preschool age.
The study was conducted in the outskirts of Harare, Zimbabwe at 2 peri-urban areas of Chitungwiza and Epworth. Chitungwiza city is located 15 kilometers southeast of the capital of Zimbabwe, Harare. It is a residential dormitory for the capital and was formed in 1978 by amalgamation of three townships Zengeza, Seke and St Marys. It has a young population of 400 000. Epworth is located south of the capital; within 20 kilometer radius. It is administered by a rural administrative board. However, following a Government directive, prior to the study period, families perceived to be living in urban slums of Chitungwiza and Epworth were forcibly evicted to the rural areas in a programme called Murambatsvina (Operation Restore Order) [26]. Consequently the follow up of the children was limited by the dispersion of town communities. Some families could not be located due to lack of forwarding addresses.
The clinics of St Mary`s, Seke and Epworth were selected as study sites based on the number of patients attending the health centers and feasibility of carrying out clinical research. The clinics offer a wide range of family health services and are used mainly by the low income population.
Participants in this study were 278 preschool children born to mothers participating in a national PMTCT program and followed up to 5 year for growth and neurodevelopment assessment. The paediatric study power determination was calculated using the Power and Sample size calculation (PS program version 3.1.2.2014, WD Duport &WD Plummer, Nashville, USA.). Based on an estimated prevalence of cognitive impairment among normal school age children 6-8 years of 3% from a previous study conducted in the same region (20) and an anticipated level of cognitive impairment in HIV infected children of 16%, a sample size of a 40 HIV infected children was anticipated to give the study power of 80%. Our sample size of 25 HIV infected was small and therefore not powered enough to infer a statistically significant relationship between paediatric HIV infection and cognitive function at preschool age.
The inclusion criteria were children who are aged 3½ years and above, infected with HIV, infants exposed uninfected with HIV, and infants unexposed uninfected with HIV whose mothers were participating in the BHAMC study. Excluded were infants who had central nervous system (CNS) pathology due to causes other than HIV and in the case of a twin pregnancy, the second twin. Informed written consent was obtained from the mother.
The Kaufman`s short form of the McCarthy scales of Children `s Abilities (MSCA) was administered cross sectional to determine the cognitive development of the children. Trained research staff blinded to the HIV status of the child, in the presence of the parent or guardian assessed the children.
The McCarthy Scales of Children’s Abilities is an assessment tool that was developed for children of ages 2½ through to 8½ years. It assesses children’s present level of functioning in intelligence and motor ability with the aim to identify possible developmental delay in different skill areas. Kaufman`s short form of the McCarthy Scales of Children`s Abilities was adapted in accordance with the MSCA manual instructions. It consists of a six-test abbreviated version of the General Cognitive Scale and gives a proportional representation to the Verbal, Perceptual-Performance, Quantitative and Memory scales which correlates substantially with the entire McCarthy scales. It serves as a screening instrument for a wide variety of mental functions and is brief to administer and score (20 to 25 minutes).The six tests items in the Kaufman short form are Puzzle Solving, Word knowledge, Numerical memory, Verbal fluency, Counting and sorting and Conceptual Grouping. The reliability of the estimated GCI was .90 in the standardization sample and was .71 in this sample.
In this study cognitive ability was expressed as a GCI score based on age specific normative data among American children and classified as follows: GCI ≥ 120 superior intelligence; 85 to 119, average to high average; ≤ 84 low intelligence. All children scoring below 64 were aggregated to 64.
Based on a pilot study (n=21), culturally appropriate modifications to some of the test items were made to make the battery of tests applicable. Test content and format was preserved. Test items were examined individually to establish which pictures and items were recognizable and to evaluate clarity of instructions. As a result of piloting we substituted some items with familiar materials. Translations of all instructions, objects and pictures were made into the local Shona language. During the assessments establishing rapport with the participants was emphasized. Detailed preparatory instructions and explanation of errors and successes seemed to enhance performance. All assessments were carried out in Shona by examiners who were fluent in the language in offices situated at the quiet end of an outpatient department. Data were reviewed regularly to optimise quality control and inter -observer reliability specifically with the scoring criteria. Inter–tester reliability among study examiners was maintained by centralized training of all the project examiners by a senior clinical psychologist. As the MSCA was administered only once, the test retest measure was not assessed. Inter rater reliability was enhanced by strict adherence to the scoring system.
Since this instrument had not been standardised in Shona speaking children, scores based on American test norms may not necessarily be equivalent for Zimbabwean children.
Three proxy socioeconomic measures were used, maternal education employment status and availability of financial subsistence (ability to subsidize income even when not formally employed). Maternal education was operationalized as number of years the mother attended formal education. Maternal age was classified into two groups: less than 20 years versus 20 years and older; marital status was classified into two groups: married/cohabitating versus single/divorced/widowed; Maternal HIV status classified into two groups: HIV infected versus HIV uninfected. Child anthropometric measurements: weight in kilograms with one decimal place was measured using hanging Salter scale, which was calibrated before use; head circumference in centimeters was measured by a non-stretchable tape measure and vertex to heel recumbent length was measured with the infant in a supine position on a paediatric length-board stadiometer. Birth weight of less than 2500 grams was defined as low birth weight and head circumference of <-2 standard deviation SD below the mean for age was defined as a small head. For infants, anthropometric indicators of nutrition status were determined from weight and height data. Infants with a Z score of <-2 were defined as undernourished, with a Z score <-2 for weight for age and height for age defining underweight and stunting, respectively [27]. Moderate to severe stunting was defined as the presence height for age of -2 to -3 Z score.
Single dose nevirapine prophylaxis compliance referred to both the HIV infected mother and her infant each receiving a single dose of nevirapine (2 mg/kg body weight) within 72 hours of delivery as was the standard of care during the study period.
Infant health status was classified according to WHO`s Intergrated Management of Childhood Illness guidelines [28].
Infant HIV status in infancy was categorised into 4 groups: HIV infected, HIV exposed but uninfected and HIV unexposed uninfected and child with unknown HIV status (child not screened for HIV). The children with unknown HIV status were born to HIV infected or uninfected mothers, whose parents or guardians did not give consent for HIV testing.
Infant blood samples were processed and tested for HIV with DNA polymerase chain reaction (PCR) 1.5 (Roche Diagnostic, Indianapolis, IN, USA) if the infant was aged less than15 months and with the rapid HIV antibody tests, Determine (Abbott Diagnostics, Abbott Park, IL, USA) and Oraquick (Abbott Diagnostics) if the child was aged 15 months or older. A child was considered to be infected with HIV if HIV DNA PCR test was positive for those aged less than 15 months or HIV antibody test positive for those 15 months or older.
Cognitive function was classified according to the sum of the weighted scores in the six tests items of the Kaufman short form: Puzzle Solving, Word knowledge, Numerical memory, Verbal fluency, Counting and sorting and Conceptual Grouping. The General Cognitive Index (GCI) was computed from the total score. A score of 84 and below (-1 SD below the Mean) was selected as the cutoff point for cognitive impairment in this cohort. All analysis was conducted using SPSS for windows (Rel 12.0.1, 11 Nov 2003, Chicago, SPSS, inc). Statistical analysis consisted of comparisons of proportions using the Chi square and Fischer’s exact tests for categorical data. Comparison of group averages was based on analysis of variance (ANOVA) when normality of data was assumed otherwise Kruskal Wallis test was used. Pearson correlation was used to determine the association between the BINS scores and MSCA GCI scores. All tests were being 2 tailed. A P-value of ≤ 0.05 was be considered statistically significant and only calculated for non-missing data. Logistic regression was employed to generate odds ratio and predict cognitive impairment. All variables were categorized in the multivariate model. Maternal age was categorized as <20 or ≥ 20 years. Maternal education was categorized as “less than primary school if she did not complete 7 years of school, “primary” if she completed 7 years and secondary or higher if she had more than 7 years education. Maternal income was categorized into <30 US dollars or ≥ 30 US dollars. Birth weight was categorized into <2500 rams (low birth weight), ≥ 2500 grams (normal birth weight), Height, Weight, head circumference were categorized into abnormal (<2 SD of mean for age and normal (>SD of mean for age).The number of surviving HIV infected children in this sample determined relatively small sample size (n=25). Child had unknown HIV status if the parent or guardian did not give consent for child HIV testing.
The study was approved by the Norwegian regional committee and Medicine Research Council of Zimbabwe. Written consent for the study was obtained on behalf of the children from their primary care-givers.
Table 1 presents a comparison of the children included in the study cohort at age 3-5 years stratified according to HIV status group with respect to a number of demographic variables. No differences were found among the three groups with respect to maternal age, education, marital status and income. The 278 children were evenly divided by gender.
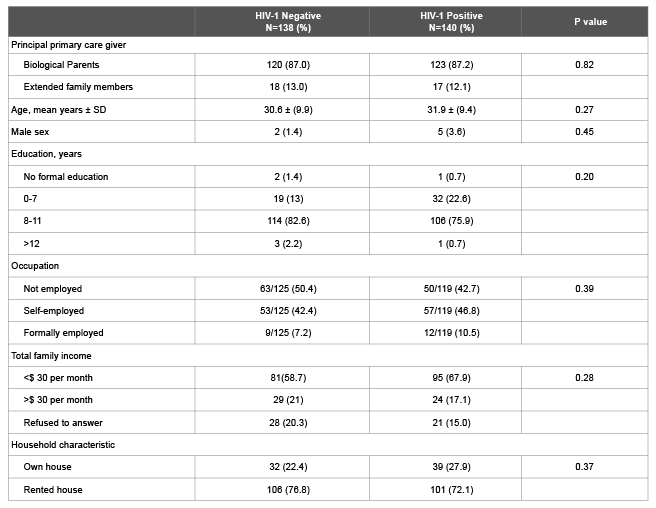
Table 1: Description of child care giver at the examination of 278 preschool children by Maternal HIV status
The age corrected GCI scores for the 278 children ranged from 64 to 136 (mean 104; SD 13.9). The means in GCI for the 4 different groups according to the child’s HIV status were all within the normal range compared to the standardization population (Table 2). Contrary to our hypothesis, there were no significant group difference in the global cognitive function between the HIV infected and HIV uninfected children. However, some focal differences were evident in word knowledge, numerical memory, verbal fluency, counting and sorting and conceptual grouping subtests between the HIV exposed uninfected and HIV unexposed uninfected groups. Exposed uninfected children scored lower scores compared to unexposed uninfected children. Child HIV clinical stage was not associated with lower GCI scores in this cohort (Table 3).
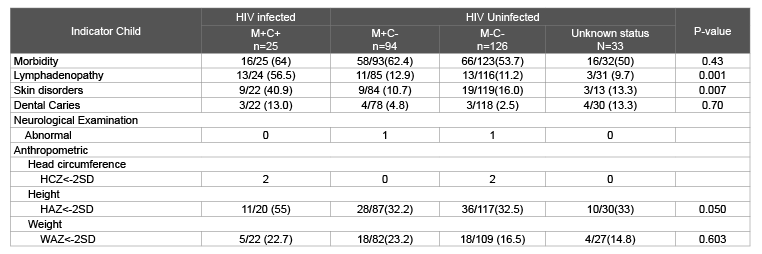
Table 2: Comparison of clinical examination of 278 preschool children stratified by maternal [M] and child [C] HIV status: HIV positive (M+C+), HIV exposed
uninfected (M+C-) and HIV unexposed uninfected (M-C-)*.
*The 2 children with abnormal neurological examination had congenital ptopsis, and were referred for further investigations.
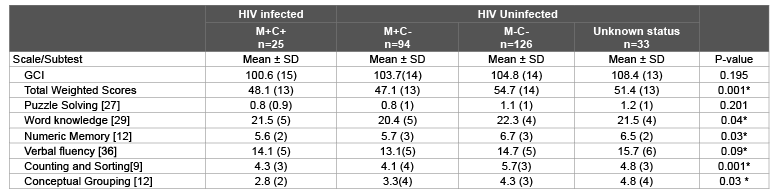
Table 3: Age adjusted Subtest scores and GCI according to Kaufman short form MSCA, means, standard deviations, for children aged 3 ½ to 5 ½ years
by child HIV status.
[ ] denotes optimum weighted score for each subtest
* Post hoc test: HIV unexposed uninfected versus HIV exposed uninfected
Based on the McCarthy Scales Children Abilities estimated General Cognitive Index, twenty 24 children (9%) had a score less than 1 SD of the mean for age and were classified as cognitive impaired, 204(73%) had a normal cognitive score, and 50 (18%) had a superior cognitive score.
Table 4 shows child cognitive impairment was significantly associated with, maternal younger age, low family income and living in rented lodgings. Children who were stunted or had a small head size for age, showed a trend towards being more cognitive impaired compared to children with normal growth though not statistically significant.
This study provides data on Zimbabwean preschool aged, HIV type 1 vertically infected children followed from birth for neurodevelopment and whose mothers participated in a national PMTCT program. The primary finding was that preschool aged vertically infected HIV children showed normal global cognitive function on Kaufmann short form of MSCA. This contrast with our hypothesis that there would be significant group difference in the global cognitive function between the HIV infected and HIV uninfected children with HIV infected performing lower GCI scores. Studies conducted early in the HIV epidemic described children characterized by lower scores on global function related to the degree of encephalopathy in infancy [29-33]. Our finding confirms results from previous research where children without AIDS defining illness performed as well as uninfected children [34]. However, the children in that study were also receiving anti-retroviral treatment [35], unlike in this series where HIV infected children were predominantly asymptomatic and not on treatment.
Significant focal differences were evident in the subtests of the MSCA, in particular Word Knowledge, Numerical Memory, Verbal Fluency, Counting and Sorting and Conceptual Grouping between the HIV exposed uninfected and HIV unexposed uninfected children, but no difference between the infected and uninfected children. These subtests represent verbal, perceptual and quantitative performance on the full scale McCarthy. Specific cognitive impairments associated with HIV infection have been identified in previous research. These include visuospatial, motor integration, language impairment, sequential processing and adaptive functioning [36]. Boivin in DRC conducted research on asymptomatic HIV infected children and had with similar findings to our study. In that series their findings indicated lower scores in sequential processing, spatial memory and auditory and visual recall [37]. The poorer performance by HIV exposed uninfected children in this study on the subtests of MSCA may be a reflection of the harsh home environment defined by having close family members, particularly the mother succumb to HIV related illness.
Partial explanation for the pathophysiolgy on the relation between HIV and cognitive function has been provided by neuro-imaging research. Neuro-imaging studies conducted on HIV infected children already on highly active antiretroviral therapy (HAART) have documented changes in the immature central nervous system, in particular the pyramidal tract, basal ganglia, frontal and prefrontal areas of the brain [13,38]. Myelinopathy resulting from HIV infection has been implicated in cognitive, visuomotor, language and behavioral delays which become more prominent over time [39]. Since no neuro-imaging was assessed in any of the children in this cohort, we were unable to determine whether there are structural brain changes associated with cognitive deficits.
Neurological compromise associated with paediatric HIV infection is hypothesized to be related to the direct effects of the virus on the neural tissues [39]. The resultant CNS dysfunction manifests as HIV related encephalopathy of the progressive or static type [35,39]. Progressive encephalopathy, the worse type, is associated with impaired brain growth, loss of developmental milestones, spasticity and behavioral disorders. In static encephalopathy, there is no insidious deterioration of attained milestones; however acquisition of new skills occurs at a much slower rate for the child’s age. Cognitive impairment in children with static encephalopathy has been reported to range from low, average to high. Static encephalopathy as opposed to progressive encephalopathy has been observed in older children [39]. We did not observe significant differences in motor deficits in this study. It is likely that the more affected infants with progressive encephalopathy from our cohort probably did not survive into childhood.
Our cohort represents children who survived to preschool age in the absence of highly active antiretroviral drugs. Various factors have been put forward to explain the survival selection including maternal host factors, infant host factors, viral phenotype, viral load CD4 counts, timing of transmission and disease stage. Infants with intrauterine infection were documented to have rapid disease progressive than children with intra or post-partum infection [40,41]. The child survivors in this study may reflect those children with the less aggressive disease.
In this study, 24 preschool age children (8.6%) were identified as cognitive impaired: 8.3% among the HIV positive children; 7.4% among the HIV exposed uninfected; 12% among the HIV unexposed uninfected and 9.1% among those with unknown status. The small sample size of HIV infected children might have masked the effect of HIV on cognitive performance in this study.
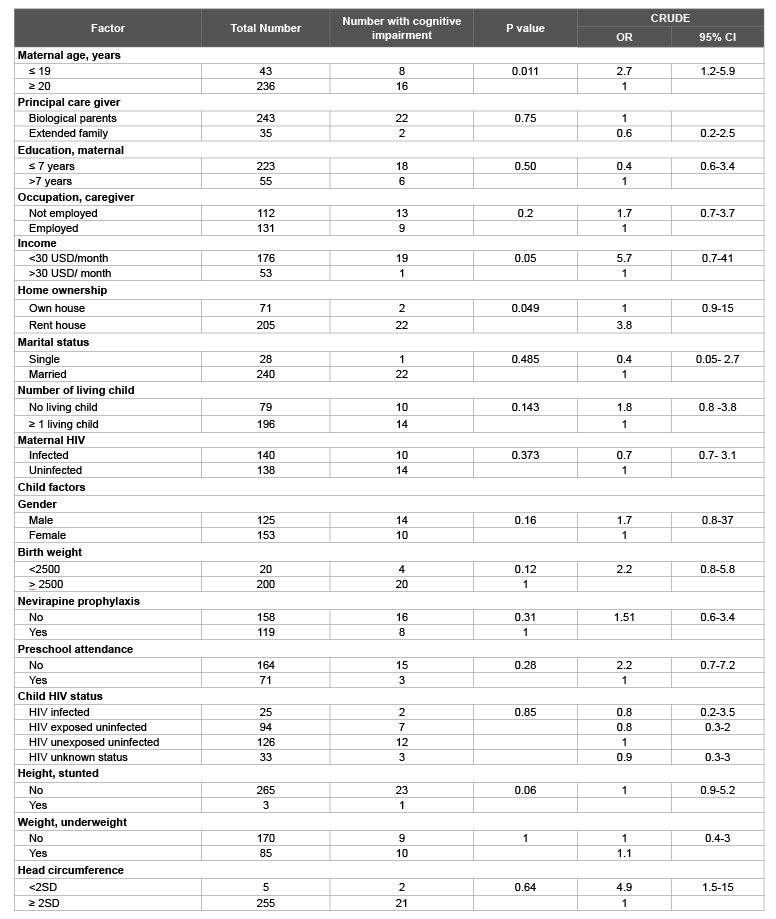
Table 4: Assessment of cognitive impairment in children aged 3 ½ to 5 ½ years (n=24) and its association with child care giver socioeconomic characteristics. Cognitive impairment defined by GCI score<1 standard deviation of mean
Family income of less than 30 US dollars per month, teenage mother and not owning a house was associated with cognitive impairment in this cohort. Proxy measures for socioeconomic status (house ownership and income less than 30 US dollars per month) in this study were significantly associated with cognitive impairment. Research has implicated a relation between socioeconomic status and cognitive ability in children. A longitudinal study conducted in Ecuador investigating the effect of iron deficiency on cognitive function found an association between low socioeconomic status and cognitive scores [42]. A study by Coscia et al. [43] established that the quality of the home environment mediated the relationship between socioeconomic status and child cognitive function while a study conducted in Kenya among children under 3 years living in poverty reported anthropometric measures such as height and weight as mediators of the relationship between socioeconomic status and psychomotor development.
Regrettable we did not assess the effect of home environment on cognitive function in this study due time and financial constraint. Based on a commonly used scale The Home Measurement of the Environment Inventory [44], it is possible to observe and evaluate home organization, play materials, parental involvement and variety of stimulation each child receives. These provide valuable information on provision of cognitive stimulation or education in the home. Only 71 (25.5%) of the children in this cohort were attending formal preschool despite early child learning services being offered for free at public schools in Zimbabwe.
Forty six children (16%) in this cohort were underweight. World Health Organisation, in 1999 estimated the prevalence of underweight in Zimbabwean under 5 year olds at 11.2%. In a study, conducted in Zimbabwe, investigating seasonal variability of the prevalence of underweight within a clinic based growth monitoring programme, the prevalence of underweight among children less than 5 years ranged 10.7 to 11.2. Our prevalence of underweight is higher than the estimated national figure. Possible explanations for the discrepancy is the current worsening in country`s economic environment resulting in less family resources being allocated for nutritional needs. Most families are either infected or affected by HIV infection, which may result in funds being channeled towards the caring of the sick parents or guardians at the expense of feeding the family. In this study underweight was not associated with cognitive impairment, which may be due to the small sample size which made the study weakly powered to detect small differences between groups. Studies in other developing countries have different findings [45-48] where being underweight was related to lower cognitive scores.
Although asymptomatic surviving HIV infected children have normal cognitive function compared to their uninfected peers, they are more susceptible to other childhood infections and nutritional deficiencies. The overall prevalence of cognitive impairment (8.6%) in this cohort was high underscoring the need to address those factors the negatively influence child development socio-economic potential as adults is to be realized [49,50].
The study had the following limitation. We used an adapted western tool to screen for cognitive function. The MSCA was developed and validated in the United States and there is no normative data for MSCA in others societies. There is no gold standard cognitive assessment tool standardized for Shona speaking Zimbabwean children. Adaptation we made to the MSCA might have influenced the reliability of the test score which might have underestimated the strength of the relationship between GCI and HIV infection at age. It is possible that some cultural bias in the MSCA still remained. The computed GCI from the subtests of MSCA may not be the most sensitive and specific test to identify subtle differences in the global cognitive performance preschool children. Furthermore MSCA`s the index scores do not allow differentiation between delay in achieving new milestones and losing previously acquired milestones. The follow up of the children was limited by the dispersion of town communities that took place prior to this follow up study. Moderate proportions of children were lost to follow up and this might result in biased estimates of mean GCI. Since the two groups (participants and those lost to follow up) were similar in the proportion of children born to HIV infected mothers, it is unlikely that this would lead to a strong bias in the means. The timing of child HIV infection was not examined in this study. This might provide answers to the variation in the pattern of cognitive function at preschool age. The effect of home environment on cognitive function was not studied due to financial constraints.
The strength of this study is its large sample size of children who were followed up in a national PMTCT program: HIV unexposed uninfected, HIV exposed infected and HIV infected.
The current study has proved that it is possible to screen preschool children for cognitive impairment in resource limited settings utilizing simple screening tools such as Kaufmann short form of MSCA.HIV infected children who survive to preschool age did not manifest lower cognitive scores than established child norms. The prevalence of cognitive impairment in this study was 8.6% and was more frequent in HIV uninfected children. Chronic malnutrition as indexed by stunting was associated with poor cognitive function at preschool age. BINS if performed in the first 6 months of life, was predictive of later cognitive performance particularly in HIV infected children. Therefore comprehensive interventions to prevent childhood HIV and malnutrition could result in significant improvement in cognitive function and later academic achievement.
Download Provisional PDF Here
Article Type: Research Article
Citation: Kandawasvika GQ, Mapingure PM, Pazvakavambwa IE, Stray-Pedersen B (2016) Neurocognitive Consequences of HIV in Preschool age Children: A Brief Report. J HIV AIDS 2(2): doi http://dx.doi.org/10.16966/2380- 5536.122
Copyright: © 2016 Kandawasvika GQ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access