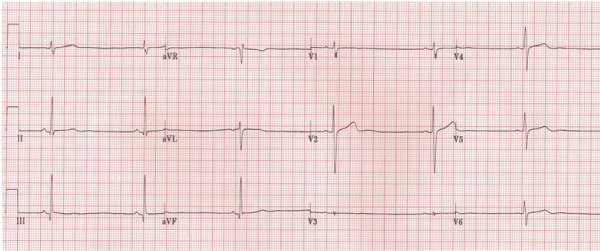
Figure 1: ECG shows sinus bradycardia.


Zaid Altheeb1 Chirag Rana2 Medhat Zaher1 Emile Doss1 Fayez Shamoon1 Wilbert S Aronow3*
1Department of Cardiovascular Medicine, New York Medical College, Paterson, New Jersey, USA*Corresponding author: Wilbert S Aronow, Cardiology Division, New York Medical College, Macy Pavilion, Room 141, Valhalla, NY 10595, USA, Tel: (914) 493-5311; Fax: (914) 235- 6274; E-mail: wsaronow@aol.com
Background: Amlodipine has not been previously reported to cause ventricular tachycardia.
Case Report: A 52-year-old patient developed symptomatic sinus bradycardia and multiple episodes of symptomatic polymorphic ventricular tachycardia attributed to amlodipine which resolved after cessation of amlodipine.
Conclusion: Although amlodipine was assumed to cause the bradycardia, there is no method to prove whether amlodipine was a direct cause of the symptoms. This could be estimated if the same symptoms occur after restarting amlodipine.
Amlodipine; Bradycardia; Ventricular tachycardia
Polymorphic ventricular tachycardia (VT) is a type of ventricular tachycardia that has a constantly changing beat to beat QRS complex. Prolongation of the QU interval by hypokalemia or hypomagnesemia or prolongation of the QT interval by drugs, especially antiarrhythmic drugs, can induce this cardiac arrhythmia. Treatment is directed at termination of the arrhythmia and prevention of future episodes from occurring.
Calcium channel blockers (CCBs) are among the most widely used drugs in cardiovascular medicine with roles not only in hypertension but also in angina pectoris and (for some CCBs) tachyarrhythmias. Amlodipine is a dihydropyridine medication that relaxes blood vessels and improves blood flow. Amlodipine belongs to a group of medications known as dihydropyridine-type CCBs. This drug is used to treat hypertension or angina pectoris [1]. Previous studies have not shown any significant effect of amlodipine on heart rate.
VT has not been reported as a side effect of amlodipine. We report a case of a patient who presented with symptomatic amlodipine-induced sinus bradycardia, bradycardia-induced QT interval prolongation, and subsequent polymorphic VT.
A 52-year-old female patient presented to the emergency department (ED) with dizziness and generalized weakness. She was found to have symptomatic sinus bradycardia with a heart rate of 30-32 beats per minute. She reported that her symptoms did not seem to be triggered by stress or physical exertion. She had no chest pain, shortness of breath, or palpitations. The patient’s medical and psychiatric histories were unremarkable except for recently diagnosed hypertension and anxiety for which she was taking amlodipine 10 mg daily for a few weeks and tizanidine 2 mg daily. She reported no significant medical history in her family and denied smoking, alcohol use, or illicit drug use.
On examination, the patient appeared lethargic. Physical examination was normal except for a regular heart rate of 30-35 beats/minute. In the ED (Emergency department), the patient was given one dose of atropine without significant improvement in her heart rate. She was admitted to a critical care unit and kept on continuous electrocardiographic monitoring. Amlodipine and tizanidine were stopped. No other medications or other treatment interventions were administered during hospitalization except for the one dose of atropine administered in the ED for sinus bradycardia.
Laboratory tests revealed negative cardiac enzymes with troponin levels consistently below 0.01 ng/ml (normal range 0.00-0.03 ng/ml). Routine laboratory tests, a comprehensive metabolic panel, and thyroid tests were within normal limits. The electrocardiogram in the ED showed sinus bradycardia with a ventricular rate of 30 beats/minute with no other abnormalities (Figure 1). On transthoracic echocardiography no evidence of valvular heart disease or abnormal wall motion was found, and the estimated left ventricular ejection fraction was reported to be 60-65%. The chest x-ray was normal.

Figure 1: ECG shows sinus bradycardia.
During her hospital course, the patient had multiple episodes of symptomatic (dizziness) polymorphic non-sustained ventricular tachycardia (Figure 2), which resolved spontaneously. Forty hours after stopping her medications, she had a normal sinus rhythm with a ventricular rate between 60-90 beats/min. Three days after admission, the patient was discharged from the hospital asymptomatic with an appropriate follow-up plan.
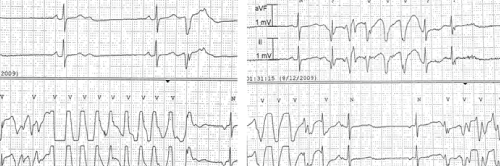
Figure 2: ECG tracings show polymorphic ventricular tachycardia.
CCBs are a class of medications that block the entry of calcium into the muscle cells of the heart and arteries [2]. The entry of calcium is critical for the conduction of the electrical signal that passes from muscle cell to muscle cell of the heart, and signals the cells to contract. Thus, by blocking the entry of calcium, calcium channel blockers decrease the force of contraction of the muscle cells, and dilate arteries.
Dihydropyridines or L-type calcium channel blockers are pyridine molecules used in the treatment of hypertension. Compared with certain other L-type calcium channel blockers (for example those of the phenylalkylamine class such as verapamil) which are known to have significant action on the heart, they are relatively vascular selective in their mechanism of action in lowering blood pressure [3].
Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine. Ex vivo studies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. Elimination from the plasma is biphasic with a terminal elimination half-life of about 30-50 hours. Steady-state plasma levels of amlodipine are reached after 7 to 8 days of consecutive daily dosing [4].
Previous studies did not define arrhythmias as side effects of dihydropyridine. Amlodipine has been evaluated for safety in more than 11,000 patients in the U.S. and foreign clinical trials. In general, treatment with amlodipine was well-tolerated at doses up to 10 mg daily. Most adverse reactions reported during therapy with amlodipine were of mild or moderate severity. In controlled clinical trials directly comparing amlodipine (N=1730) at doses up to 10 mg to placebo (N=1250), discontinuation of amlodipine because of adverse reactions was required in only about 1.5% of patients and was not significantly different from placebo (about 1%). The most commonly reported side effects more frequent than placebo are fatigue, nausea, abdominal pain, edema, flushing, palpitations and somnolence [5].
Long-acting dihydropyridines (e.g., amlodipine, extended release nifedipine, felodipine) have been shown to be safer antihypertensive drugs, in part, because of reduced reflex responses. This characteristic also makes them more suitable for treatment of angina pectoris than short-acting dihydropyridines [6].
Given our patient’s clinical presentation, recent use of amlodipine and the timeline of events and in light of the long half-life of amlodipine (30- 50 hours) compared to the relatively short half-life of tizanidine (2 hours), our major finding in this patient is that long-acting dihydropyridines like amlodipine can also be associated with profound bradycardia and subsequently life-threatening ventricular arrhythmias. Atropine is a very rare cause of polymorphic VT [7]. However, the single dose of atropine administered in the ED was not attributed as the cause of the multiple episodes of polymorphic VT that developed during hospitalization. Although amlodipine was assumed to cause the bradycardia, there is no method to prove whether amlodipine was a direct cause of the symptoms. This could be estimated if the same symptoms occur after restarting amlodipine.
Dihydropyridines used to lower blood pressure or relieve chest pain in patients with angina pectoris angina can be associated with lifethreatening bradycardia and polymorphic ventricular tachycardia. The present case report discusses these adverse side effects induced by amlodipine. A major limitation of this case report is that the patient was not challenged with seeing whether these adverse effects would recur after restarting amlodipine.
Download Provisional PDF Here
Article Type: Case Report
Citation: Altheeb Z, Rana C, Zaher M, Doss E,Shamoon F, et al. (2016) Amlodipine-induced Ventricular Tachycardia-A Case Report. J Hear Health 3(1): doi http://dx.doi.org/10.16966/2379-769X.129
Copyright: © 2016 Altheeb Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access