Abstract
Purpose: We examined the risk factors affecting excessive weight loss at 1 year after gastrectomy in patients with early gastric cancer.
Methods: 139 cases of early gastric cancer were examined. We examined by logistic analysis the risk factors that affect the excessive weight loss at 1 year after gastrectomy as classified by the BMI (<18.5 (thin), ≥ 18.5-<25 (normal), ≥ 25 (obese).
Results: When stepwise selection and multivariate logistic regression analysis was conducted with excessive weight loss as the dependent variable, excessive weight loss in the perioperative period, total gastrectomy, incidence of postoperative complications after gastrectomy, were found to be associated with weight loss.
Conclusion: Excessive weight loss rate at 1 year after surgery was affected by excessive weight loss in the perioperative period and total gastrectomy, incidence of postoperative complications after surgery.
Keywords
Weight loss after Gastrectomy; Early gastric cancer; Body mass index
Introduction
In Japan, the results of surgical resection for early gastric cancer are generally favorable as demonstrated by the five-year survival rate of 94.3% in patients with mucosal cancer and that of 89.7% in those with submucosal cancer [1]. Body weight loss caused by post-gastrectomy syndrome is a common finding after gastric cancer surgery [2]. Body weights are then maintained without any significant changes until 12 months after surgery [3]. The postoperative OS curves of patients were grouped by percentage weight loss after gastrectomy. Among the patients who survived more than 1 year after gastrectomy, patients with >10% weight loss had the worst survival compared with the other patients [4]. Body mass index (BMI) at 1 year after surgery was associated with BMI at the baseline [4]. The body weight loss of those patients with advanced gastric cancer was influenced by cachexia syndrome [5]. As the majority of patients with advanced cancer experience cancer anorexia-cachexia syndrome with weight loss, it has an object only to the early gastric cancer.
We examined the factors that affect the rate of weight loss at 1 year after gastrectomy in patients with early gastric cancer. The weight loss rate viewed separately was classified by BMI (<18.5, ≥ 18.5-<25, ≥ 25) at 1 year after gastrectomy. We defined cases as showing a decrease in more than the 75 percentile. We examined by logistic analysis the background factors that affect the rate of weight loss 1 year after surgery.
Patients and Methods
Patients
Of the139 patients (M:F 92:47, mean age 71 years) who underwent surgery for initial early gastric cancer in our department from January 2009 to January 2015, 59 patients had mucosal cancer and 80 patients had submucosal invasion. 7 patients had lymph node metastasis and No patient had distant metastasis (Table 1). 15 cases who were intended for curative resection were excluded as untraceable, due to reasons such as changing hospital. On admission, patients were classified by BMI, thin (<18.5) 16 cases, normal (≥ 18.5-<25) 92 cases, to classify obesity (≥ 25) 31 cases (Table 2).
|
n=139 |
Gender (Male : Female) |
66.2% (n=92) : 33.8% (n=47) |
Age |
71‡ |
(32-89)† |
BMI (kg/m2) |
preoperative |
22.5 kg/m2‡ |
(16.4-35.7)† |
|
the day discharged from hospital |
20.9 kg/m2‡ |
(15.5-33.3)† |
1 year after gastrectomy |
20.2 kg/m2‡ |
(14.7-33.3)† |
%VC |
104.7%‡ |
(41.7-135.0)† |
FEV1.0% |
74.8%‡ |
(36.5-100.0)† |
PNI |
49.4 ‡ |
(32.8-71.6)† |
mGPS(A/B/C/D) |
79.1% (n=110) /2.9%(n=4) /7.2%(n=10) /10.8%(n=15) |
NLR |
2.1‡ |
(0.6-7.7)† |
T T1a (M)/ T1b (SM) |
42.4%(n=59)/57.6%(n=80) |
N N0/N+ |
95.0%(n=132) /5.0%(n=7) |
M M0 |
100%(n=139) |
Table 1: Patient characteristics at the time of admission.
‡ mean † range
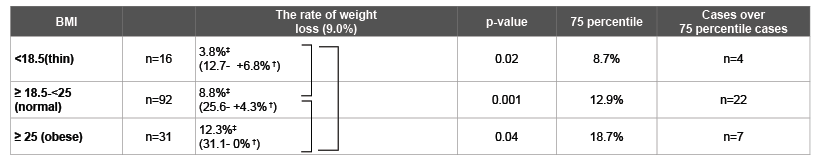
Table 2: The rate of weight loss at 1 year after gastrectomy in patients with early gastric cancer: Exceed cases over the 75 percentile (8.7%; thin, 12.9%; normal, obese 18.7%) where 33 cases ( 4 cases; thin, 22 cases; normal, 7 cases; obese).
‡ median † range
Kruskal- Wallis p=0.0003, Steel-Dwass test All patients 9.0%‡(31.15- +6.82% †)
Methods
The weight loss rate viewed separately was classified by BMI (<18.5, ≥18.5-<25, ≥ 25) at 1 year after surgery and in the perioperative period (Tables 2 and 3). We defined cases as a decrease of more than the 75 percentile (Tables 2 and 3). Analysis of clinicopathological factors associated with body weight loss at 1 year after surgery was performed. Clinocopathological categories were as follows: gender (Male/Female), age (≥ 80/<80), weight loss in a perioperative period (excessive/Normal), gastrectomy (Distal/Total), lymphadenectomy (40), modified Glasgow Prognostic Score (mGPS) (score 0-1/2), neutrophil lymphocyte ratio (NLR) (≤ 3/>3), postoperative complications (Yes/No), Laboratory data tested the values immediately before surgery. Pulmonary dysfunction was defined as less than 80% of the percentage vital capacity (% VC) or less than 70% of forced expiratory volume percentage per second (FEV1.0%). Scoring of mGPS was as follows: 0; CRP ≤ 1.0mg/dl, 1 CRP>1.0 mg/dl, 2; CRP>1.0 mg/dl and Alb<3.5 g/dl. NLR, body weight loss (excessive) was divided by the 75 percentile from the cumulative frequency distribution at 1 year after surgery and in a perioperative period. Perioperative was defined as up to hospital discharge date from preoperative.
The rate of weight loss at 1 year after gastrectomy was defined as follows: (preoperative body weight-lean body weight 1 year after gastrectomy) × 100/preoperative body weight.
The weight loss in a perioperative period was defined as follows: (preoperative body weight-lean body weight at the time of hospital discharge) × 100/preoperative body weight. They were given nothing orally for 1 days after the operation. On postoperative day 1, the patients began to drink water. Then a liquid diet and soft rice porridge diet were commenced on days 2 after, respectively, with peripheral parenteral nutrition being given accordingly.
Judgement of complications: The incidence of complications was defined when a grade Ⅱ or more of JCOG complication criteria according to Clavien-Dindo classification [6] was noted (Table 3).
|
n |
% |
Overall complication |
n=22 |
15.8% |
Intraabdominal abscess |
n=4 |
2.9% |
Intraabdominal bleeding |
n=1 |
0.7% |
Anastomotic leakage |
n=4 |
2.9% |
Wound infection |
n=3 |
2.2% |
Inflammation of the gallbladder |
n=1 |
0.7% |
Urinary tract infection |
n=1 |
0.7% |
Pulmonary atelectasis |
n=3 |
2.2% |
Pneumonia |
n=4 |
2.9% |
Ileus |
n=4 |
2.9% |
Chylothorax |
n=1 |
0.7% |
Table 3: The incidence of complication: Complications were defined when
a gradeⅡor more of JCOG complication criteria according to Clavien-
Dindo classification.
There is some overlapping
Statistical analysis
Statistical analyses were performed using SAS ver.9.2 (SAS Institute, Inc., Cary, NC, USA). Analysis of variance for continuous variables was used for each group of percentages of body weight loss. Univariate and multivariate logistic regression analyses were performed to identify factors associated with percentage body weight loss at 1 year after surgery. Variables for inclusion in the multivariate analysis were selected by a stepwise procedure with all variables (stepwise forward selection method with entry and stay criteria both set to p=0.15). Other statistical methods were analysed by Tukey-Kramer method (honestly significant difference test). p <0.05 was considered statistically significant.
Results
Weight loss rate at 1 year after surgery
The rate of weight loss in a perioperative period patients was 6.3% (4.4%; thin, 6.5%; normal, 8.9%; obese) in all cases. Cases over the 75 percentile (5.7%; thin, 10.3%; normal, obese 11.0%) were 33 cases (4 cases; thin, 22 cases; normal, 7 cases; obese). There were significant differences among those patients (Table 2).
Univariate logistic regression analyses
In univariate logistic analysis, the significantly high incidence of the weight loss rate of at 1 year after gastrectomy was excessive weight loss in the perioperative period 10.1 (95% CI 4.21-25.5), total gastrectomy 11.1 (95% CI 3.39-43.36), D2 lymphadenectomy 3.24 (95% CI 1.18-8.74), mGPS 26.25 (95% CI 2.06-20.29), incidence of postoperative complication 21.5(95% CI 7.40-73.1) (Table 4).
|
|
n |
% |
Odds ratio |
95% confidence
interval |
p-value |
Gender |
male |
92 |
(-66.2) |
1.23 |
0.54-2.96 |
0.63 |
Age |
≥ 80 |
24 |
(17.3) |
0.4 |
0.09-1.28 |
0.17 |
Weight loss in a perioperative period |
excessive |
34 |
(-24.5) |
10.1 |
4.21-25.5 |
0.0001 |
Gastrectomy |
total |
14 |
(10.1) |
11.1 |
3.39-43.36 |
0.0002 |
Lymphadenectomy |
D2 |
20 |
(-14.4) |
3.24 |
1.18-8.74 |
0.02 |
Approach |
open |
63 |
(-45.3) |
1.63 |
0.74-3.61 |
0.23 |
Pulmonary dysfunction |
%VC<80 or FEV % <70
1.0 |
41 |
(-29.5) |
0.87 |
0.35-2.02 |
0.75 |
Diabetis mellitus |
Presents |
23 |
(-16.5) |
1.16 |
0.39-3.12 |
0.77 |
PNI |
≤ 0 |
14 |
(-10.1) |
1.32 |
0.34-4.29 |
0.66 |
mGPS |
2 |
15 |
(-10.8) |
6.25 |
2.06-20.29 |
0.0001 |
NLR |
>3 |
35 |
(-25.2) |
2.06 |
0.87-4.78 |
0.09 |
Postoperative complications |
incidence |
22 |
(15.8) |
21.5 |
7.40-73.1 |
0.0001 |
Table 4: Univariate logistic regression analyses: In univariate logistic analysis, the significantly high incidence of the weight loss rate at 1 year after gastrectomy was excessive weight loss in the perioperative period 10.1 (95% CI 4.21-25.5), total gastrectomy 11.1 (95% CI 3.39-43.36), D2 lymphadenectomy 3.24 (95% CI 1.18-8.74), mGPS 26.25 (95% CI 2.06-20.29), incidence of postoperative complication 21.5 (95% CI 7.40-73.1).
Stepwise selection and multivariate logistic regression analyses
A stepwise multivariate logistic regression analysis was conducted with the weight loss rate of at 1 year after surgery as the dependent variable and all other factors as independent variables, excessive weight loss in the perioperative period 12.4 (95% CI 4.02-44.3), total gastrectomy 12.1 (95% CI 2.56-67.9), incidence of postoperative complications 25.4 (95% CI 6.93-113.8), were finally selected as being associated with the weight loss rate at 1 year after gastrectomy (Table 5).
|
|
n |
% |
Odds ratio |
95% confidence interval |
p-value |
Weight loss in a perioperative period |
excessive |
34 |
(-24.5) |
12.4 |
4.02-44.3 |
0.0001 |
Gastrectomy |
total |
14 |
(-10.1) |
12.1 |
2.56-67.9 |
0.003 |
Postoperative complications |
incidence |
22 |
(-15.8) |
25.4 |
6.93-113.8 |
0.0001 |
Table5: Stepwise selection and multivariate logistic regression analyses: A stepwise multivariate logistic regression analysis was conducted with the weight loss rate at 1 year after surgery as the dependent variable and all other factors as independent variables, excessive weight loss in the perioperative period 12.4 (95% CI 4.02-44.3), total gastrectomy 12.1 (95% CI 2.56-67.9), incidence of postoperative complications 25.4 (95% CI 6.93-113.8) were finally selected as being associated with the weight loss rate at 1 year after gastrectomy.
Discussion
We have practiced a continued pre-through, post-operative nutritional education for patients with gastric cancer [7]. However, we routinely experience weight loss after gastric cancer surgery. Excessive weight loss requires attention because it may cause nutritional disorder, however, the reference value for the rate of weight loss remains unclear. In the results of our study, the rate of weight loss 1 year after gastric cancer surgery was 9.0% as compared with before surgery. By BMI, the rates were 3.8% in underweight patients, 8.8% in normal weight patients, and 12.3% in obese patients, showing lower rates of weight loss in patients with a low BMI. Yamaoka et al. [8] compared the rates of weight loss between patients with BMI<22 and those with BMI ≥ 22 at one year after gastric cancer surgery and reported the rates were 11.0% for the former patients and 18.3% for the latter patients. Also, Matsuura et al. [9] studied the changes in the rates of weight loss and nutritional status in patients with gastrointestinal cancer with BMI<18.5 and those with BMI ≥ 25 at one year after gastric cancer surgery. They reported that the rate of weight loss is lower in patients with BMI<18.5 than those with BMI ≥ 25; however, energy intake and the serum albumin level substantially decreased, deteriorating nutritional status. Based on these results, we consider that the rates of weight loss resulting in nutritional disorder vary by BMI and defined patients exceeding the 75 percentile in cumulative frequency tables for underweight, normal, and obese as patients with weight loss. Moreover, based on this definition, we examined factors affecting severe weight loss at one year. We examined by logistic analysis the risk factors that affect the excessive weight loss 1 year after gastrectomy. Total gastrectomy, incidence of postoperative complications, excessive weight loss in perioperative period were independent risk factors.
The extent of gastrectomy is directly associated with digestion and absorption impairment and retention function, affecting meal intake volume and weight [10]. Therefore, the rate of weight loss is stabilized at approximately 10% in patients who underwent distal gastrectomy 3 months after surgery onward, whereas weight loss continues in patients who underwent total gastrectomy for 1 year, and the rate of weight loss is high at approximately 18% [11]. In the comparison between total gastrectomy and distal gastrectomy, decrease in food consumption volume associated with disappearance of the function of the remaining stomach reportedly results in weight loss in patients who underwent total gastrectomy,which was consistent with the results of our study.
In addition to total gastrectomy, postoperative complications and excessive weight loss in a perioperative period were risk factors. Body weight loss caused by surgical morbidity is a common finding after gastric cancer surgery [2]. The weight loss in a perioperative period may be associated with oral intake disability caused by postoperative complications. Preoperative nutritional status is related with postoperative complications [12]. There is a concern over weight loss associated with oral intake disability before admission and fasting for preoperative tests. In fact, the rate of weight loss in a perioperative period has been reported to be approximately 5 to 6% [11], and the frequencies of onsets of complications and immunity vary by the rates of weight loss [10]. Kuno et al. [13] confirmed that weight loss during 1 month from before surgery continued.
Then, we should consider the excessive weight loss in a perioperative period. Various efforts to prevent perioperative weight loss have been reported, such as the administration of a nutritional supplement containing eicosapentaenoic acid (EPA) [2], enhanced recovery after surgery (ERAS) protocol [14], and highly nutritious between-meal snacks.
Total gastrectomy, incidence of postoperative complications, excessive weight loss in perioperative period were risk factors of excessive weight loss 1 year after gastrectomy in patients with early gastric cancer. For those patients, we wish to reinforce team medical care to implement a continuous approach from the initial examination and enhance continuous dietary instructionfrom the preoperative to postoperative period.
Conclusion
Excessive weight loss in perioperative period, total gastrectomy, incidence of postoperative complications were independent risk factors of excessive weight loss at one year after gastrectomy in patients with early gastric cancer.
Conflict of Interest
All the authors have read the manuscript and have approved this submission. And there are no conflicts of interest.
Article Information
Article Type: Research Article
Citation: Sagawa M, Katsube T, Murayama M, Yamaguchi K, Konno S, et al. (2016) Risk Factors Affecting Excessive Weight Loss at One Year after Gastrectomy in Patients with Early Gastric Cancer. J Gastric Disord Ther 2(5): doi http://dx.doi. org/10.16966/2381-8689.130
Copyright: © 2016 Sagawa M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 22 Aug 2016
Accepted date: 02 Dec 2016
Published date: 08 Dec 2016