
Table 1: Risk factors for gastric adenocancer
Serra Kayacetin*
Department of Pathology, Ankara Numune Training and Research Hospital, Ankara, Turkey*Corresponding author: Serra Kayacetin, Department of Pathology, Ankara Numune Training and Research Hospital, Ankara, Turkey, E-mail: skayaetin@gmail.com
All gastric carcinomas are originated from foveolar (stem/basal) cells, in practice. The most important development in this field is the detection of H.pylori’s role in the development of chronic gastritis as an important etiological factor. In most examples, they develop on the ground of intestinal metaplasia and chronic atrophic gastritis, and led by various stages of dysplasia, carcinoma in situ, and carcinoma. The incidence of gastric carcinoma shows geographical differences distribution. Especially in some countries like Japan and Columbia, the incidence of gastric carcinoma is greater than other countries.
Patients are mostly asymptomatic in the early stage. Gastric carcinomas spread into the depths of the gastric wall. Early gastric carcinoma and advanced gastric carcinoma are divided into. Adeno Ca histological grading made according to the degree of differentiation at the gland direction. Stage of the disease, condition of lymph nodes, and penetration degree of the tumor tissue in the gastric wall are important factors that determine the prognosis. Factors affecting prognosis in gastric cancer are; invasion depth (mucosal, submucosal spread, gastric wall), lymph node metastasis, and lesion size.
The five year survival rate in patients with gastric adenocarcinoma is on average 10-15%. Endoscopic mucosal resection in the treatment of early gastric cancer has been becoming more widespread in recent years and its prognosis is very good. It should be carefully examined with endoscopic ultrasonography before the endoscopy to ensure that these lesions are not overflown outside submucosa. In advanced gastric patients that do not have any chance of curative surgery, various multiple chemotherapy regimens are tried.
Gastric cancers; Etiology; Pathogenesis; Classification
Pathogenesis of the gastric carcinoma is closely associated with environmental factors [1]. Its frequency had decreased apparently in certain states like USA and United Kingdom but in others like Japan, Chile, and Italy it shows an excessive increase [2,3]. Nevertheless, there is growing body of evidence that genetic predisposition might play an important role since the disease is seen as familial in approximately 10% of the cases [4].
Many of the patients are above 50 years old but younger and even pediatric cases were also reported [5,6]. Cases that are seen at a very young age or in old people show clinicopathological differences compared to the ones that are seen in the usual age group [7].
In practice, all gastric carcinomas are originated from foveolar (stem/ basal) cells. In most examples, they develop on the ground of intestinal metaplasia and chronic atrophic gastritis, and led by various stages of dysplasia, carcinoma in situ, and carcinoma [8]. Even though it is argued that some cases are caused by the heterotypical pancreatic tissue or submucosal cysts that are fitted with some other epithelium on the stomach wall, this is a very rare condition [9].
Eighty five to ninety percent of gastric carcinoma cases are accompanied by hypochlorhydria. It is widely accepted that high intragastric pH favors the production of bacteria that reduce nitrate in the diet to nitrite and later in the presence of this nitrite, turns dietary amines into carcinogenic N-nitroso compounds [10-13]. Simultaneous existence of chronic atrophic gastritis and carcinoma is frequent but the etiopathogenetic connection between the two and the relative risk for malignancy in the presence of the first remains controversial [14]. The same thing also applies for pernicious anemia; although the development rate of carcinoma increases statistically in this condition, it is not prevalent enough to recommend close observation of asymptomatic patients [15].
The most important development in this field is the detection of H.pylori’s role in the development of chronic gastritis as an important etiological factor [16-18].
Although the tumor type that is mentioned above and characterized with epidemiological and sequential features is accepted to represent only one, albeit the most prevalent, type of gastric carcinoma. This tumor is defined as intestinal type of adenocarcinoma by Lauren [19]. The other type, i.e diffuse carcinoma, is not characterized by the above associations and appears less likely to be connected to environmental factors [20]. In fact, it has been shown that intestinal type of adenocarcinoma is the dominant form in high risk areas. Moreover, when the incidence of gastric carcinoma shows a decrease in a society, it is this microscopic type that first starts to decrease [21].
Other factors that are thought to be involved in the pathogenesis of gastric carcinoma (although in the minority) are gastric polyps, menetrier disease, gastric peptic ulcer disease, and gastric stump [22].
For other malignancies in younger patients, cases of gastric carcinoma were reported following the radiation and chemotherapy [23]. Sometimes gastric adenocarcinoma is seen simultaneously with esophageal squamous cell carcinoma [24].
Some cases of gastric adenocarcinoma are associated with EBV (lymphoepithelioma-like); the frequency of such an occurrence varies from 6-7% in Japan and Europe to 16% in USA. EBV is more prevalent in men than women and in some countries than others. It is a rule that EBV is found in lymphoepithelioma-like tumors [25]. When it does, it is seen as an early case independent of the expression of Bcl-2 and the accumulation of p53 [26].
Gastric carcinoma is the second most prevalent cancer in men, and fourth most prevalent cancer in women worldwide [27]. The incidence of gastric carcinoma shows geographical differences with an uneven distribution. Especially in some countries like Japan and Columbia, the incidence of gastric carcinoma is greater than other countries [2]. The incidence in Japan is 10 times greater than many regions of Africa, and 65 times greater than North America. The places where the incidence is high are: Finland, Poland, Iceland, Russia, Chile, China, and Japan [28]. The places where the incidence is low are: India and Uganda. Incidence is approximately 9.6/100.000 in USA, and 78.8/100.000 in men and 46.3/1000.000 in women in Japan [29]. It is the most frequent type of cancer that is the second most prevalent cancer in men and the sixth most prevalent cancer in women in Turkey, among other types. Among the cancer types seen in Turkey, gastric cancer constitutes 8% in men and 6% in women. Mortality rate from gastric cancer in Turkey is 8-9/100.000 in men and 4-5/100.000 in women. Interestingly, in societies that migrate from high risk areas to low risk areas, the risk of gastric cancer in the migrating society decreases to the level of the local community starting from the second generation. The reverse is also true, that is the societies that migrate from low risk areas to high-risk areas start to have an incidence that increases and becomes gradually similar to that of the recipient area. In some countries the risk of gastric cancer is higher in rural areas and it decreases with the urbanization. Apart from geographical difference, there is an apparent relationship between socio-economic status and gastric carcinoma. The incidence and mortality are 3 times higher in lower socioeconomic classes than the higher ones. Gastric cancer is mostly a problem of developing countries rather than the industrialized countries [30].
Generally, chronic atrophic gastritis is evaluated as pernicious anemia, intestinal metaplasia, hyperplastic gastropathy (Menetrier disease), chronic peptic ulcer, partial gastric resection, gastric polyps, precancerous lesions, and conditions [31-33,10].
Helicobacter pylori: Infection of H.pylori is forming a basis for the formation of cancer by causing chronic gastritis, atrophy and intestinal metaplasia, and creating dysplasia. H.pylori is defined as a carcinogenic agent by WHO [34].
Chronic atrophic gastritis: It takes the top place in the pathogenesis of gastric carcinoma as a precancerous lesion, especially for intestinal type carcinomas [35].
Pernicious anemia: The increase in the carcinoma incident in people with pernicious anemia is associated with the frequency of atrophic gastritis and intestinal metaplasia in the corpus and the fundus.
Menetrier disease (hyperplastic gastropathy): Menetrier disease is a gastropathy characterized with hypoalbuminemia that develops on the basis of excessive mucus secretion and hypochlorhydria, due to the (buraya bir isim gelmeli- örneğin atrophy, destruction, impairment vb) gastric surface and hyperplasia of foveolar epithelial lining. People with menetrier disease have increased risk of gastric tumor.
Intestinal metaplasia: As with gastric carcinoma, the incidence of intestinal metaplasia increases proportionally with age. Such a variety across age groups is seen more frequent and widespread in terms of the diffuse type of intestinal type carcinomas. Classifications of intestinal metaplasia are usually done according to the cell morphology in metaplastic epithelium and mucin types produced by cells. They are divided into two main groups: complete (type I) and incomplete (type II). Complete type is similar to the normal mucosa of the small intestine with brush border absorptive cells and goblet cells, and it contains sialomucin. There are two subtypes of incomplete intestinal metaplasia which is composed of goblet cells and columnar cells that can produce several mucin types. They are divided into two subgroups as Type IIa and Type IIb. Type IIa, which is also named as the goblet cell metaplasia, is characterized by cells secreting unsulfated mucin. These cells resemble normal gastric cells. Type IIb is composed of cells that are more differentiated and predominantly secrete sulfomucin. This type of metaplasia is associated with the highest risk of cancer [36].
Gastric ulcer: Chronic benign ulcers practically do not become cancerous although some ulcers that are dependent on cancer might have a benign character and respond to medical treatment. Histological distinction must be done in gastric ulcers by endoscopic biopsy. Even when the first biopsy is negative, biopsies should be repeated after the treatment and ulcer’s status should be reevaluated endoscopically.
Partial gastric resection: After the partial gastrectomy, the remaining gastric mucosa, stoma and areas closer to it are prone to rapid development of gastric atrophy and ultimately intestinal metaplasia.
Adenomatous and non-adenomatous gastric polyps: Types of the polyps that are detected in the gastric mucosa are fundic gland polyp, hyperplastic polyp, and adenomatous polyp, in descending order of prevalence. Adenomas are seen less frequently compared to other gastric epithelial polyps (non-neoplastic). Carcinoma incidence is greater in adenomas that are larger than two centimeters in diameter. Adenomas are a precursor lesion for carcinoma and may also frequently coexist with gastric carcinoma. For this reason, when a gastric adenoma is detected, it should be removed completely and it should be thoroughly sought for whether there is another accompanying lesion [37,38].
Hereditary polyposis syndromes and gastric polyps: As there is not enough data regarding the period required for the development of gastric carcinoma and the actual risk of gastric carcinoma in patients with familial polyposis syndrome, all polyps should be excised.
Gastric epithelial dysplasia: While most of mild dysplasias originating from various reasons regress, carcinoma risk increases proportionally with the severity of dysplasia in medium and heavy dysplasias. For this reason, cases with dysplasia need to be examined with periodical endoscopic biopsies.
Genetic factors: Genetic mutations, different copies of CD44, changes in p53 and c-met genes are common features in both types of carcinoma and important symptoms for helping early carcinoma diagnosis [39] (Table 1).

Table 1: Risk factors for gastric adenocancer
It has been observed that smoking, eating habits, food storage and cooking methods also affect the risk of gastric cancer. For instance; protein malnutrition, excessive amount of salt intake, smoked foods, nitrates and chemical irritants like bile acids have been associated with increased risk of gastric cancer. Helicobacter pylori infection is an important factor for gastric cancer. Diet low in fruits and vegetables and pernicious anemia are also among the etiology of gastric cancer [40,41].
Over the last 15 years, there has been an increase in the incidence of gastric cancer (adenocarcinoma of the cardia). In paroxysmal (?) adenocarcinomas 5-year survival rate is less than other localizations. Race and sex do not affect the survival and the spread of tumor during the process of diagnosis of gastric cancer.
Infection of H.pylori, feeding habits, and changes in the gastric micro environment form the basis of the pathogenesis of gastric cancer. Helicobacter pylori is one of the most discussed factors in recent years. In general, it is thought that H.pylori starts the multiple stage carcinogenesis and causes progressive chronic atrophic gastritis and dysplastic changes. In addition, the occurrence of gastric cancer in only a few people who are infected with H.pylori suggests that there should be other carcinogenic factors. In the further steps of pathogenetic process that starts with the H.pylori, microsatellite instability and genetic changes (p53 and APC/ β-Catenin) play a role. H.pylori causes epithelial dysplasia by acting a part in many steps such as the mutation of gastric epithelial DNA, increasing free oxygen radicals, changing interleukin levels, decreasing cytoprotective substances, and changing gastric microenvironment (Figure 1). It is estimated that the risk of gastric cancer is 4 times greater in infected people than the general population.
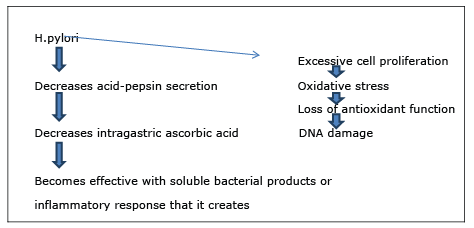
Figure 1 : H.pylori causes epithelial dysplasia.
Hypochlorhydria develops as a result of diseases such as pernicious anemia or procedures such as partial gastrectomy and is characterized by excessive intragastric accumulation of anaerobic bacteria (Figure 2). Most of these bacteria increase the levels of intragastric nitrite, n-nitroso compounds, and bile acid concentrations; they also increase the probability of dysplasia on the ground of chronic gastritis and carcinoma.
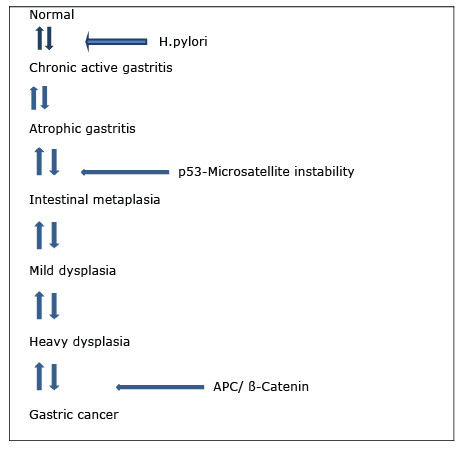
Figure 2: A Multistep pathogenetic model for gastric cancer
H.pylori prevents the balance between COX-2 (cyclooxygenase-2) cell proliferation and apoptosis, inhibits apoptosis, and is expressed abnormally within infected mucosa (Figure 3). Over expression of COX-2 is detected in positive H.Pylori gastritis, precancerous lesions, and gastric cancer [42]. It was demonstrated that COX-2 expression decreased 1 year after the eradication of H.pylori, [43]. These findings might show the early role of COX-2 expression in gastric carcinogenesis.
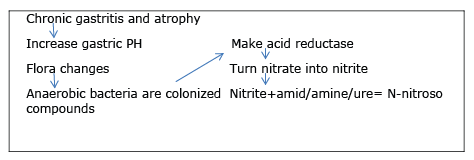
Figure 3: H.pylori infection increases the risk of chronic infection by 5-6 times.
The term Gastric carcinoma includes all malignant epithelial tumors of the stomach. However, this term is often used interchangeably with adenocarcinoma that is the most common cancer of the stomach. Gastric carcinomas might occur at any part of the stomach and both early and advanced gastric carcinomas are most frequently seen in gastric antrum and small curvature. Gastric carcinomas are seen most commonly in the distal 1/3, followed by 1/3 proximal section. Simultaneous gastric carcinoma development at many focuses is not uncommon and multiple focuses are more common in early gastric carcinomas than in the advanced gastric carcinoma.
Gastric carcinomas spread into the depths of the gastric wall. Early gastric carcinoma and advanced gastric carcinoma are divided into: Early gastric cancer is the case where malignant cells are kept limited inside mucosa and/or submucosa regardless of the lymph node involvement (Figures 4 and 5). The term early gastric cancer is a pathological one. This term does not necessarily need to be synonymous with clinically early, unexpanded, asymptomatic and small, and curable gastric cancer. This is because even if early gastric cancer is usually asymptomatic, some cases may have non-specific symptoms and lymph node involvement may be relatively extensive in some others, albeit rare.
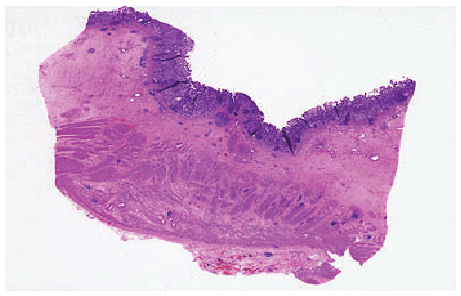
Figure 4: Early gastric cancer-Limited to mucosa/submucosa, lymph node met (+/-) cancers (muscularis externa).
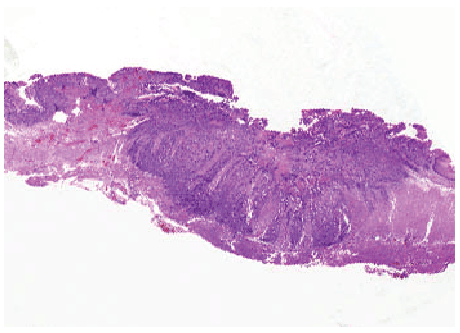
Figure 5: Advanced gastric cancer-Invading deeper than submucosa.
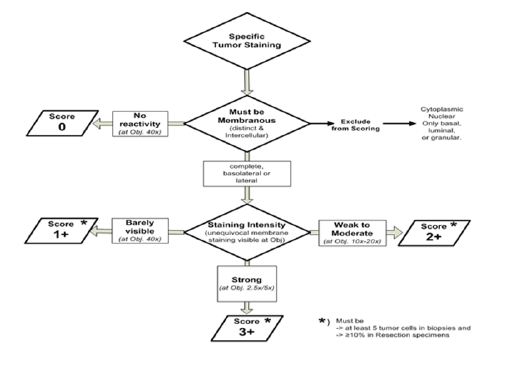
Figure 6: Intensity of immunohistochemical staining
Five to sixteen percent of gastric cancers in Europe and North America, and 20-40% of gastric cancers in Japan are diagnosed at an early stage [44].
Early gastric carcinomas are macroscopically divided into three types:
Type I (polypoid): Tumor forms a bulging from gastric mucosa towards the lumen. Tumor may have a polypoid, nodular, or villous appearance.
Type II (flat-superficial): Tumor may be bulging in the mucosal surface, be sunken or have a fin alignment. It is divided into three subgroups.
IIa: Tumor shows a slight bulge into the lumen. IIb: The tumor is at the same level with the environmental mucosal surface. IIc: The tumor is more sunken than the environmental mucosal surface.
Type III (hollow): Its appearance resembles chronic peptic ulcer. The tumor is on the edge of the ulcer or below.
Type I and type II show a well-differentiated histology; type IIb and IIc medium and varying degrees of differentiated histology; while type III shows a high proportion of poorly differentiated or undifferentiated histology [45].
Most of the cases are located in the distal stomach [46]. The lesion is multicentric in the resection specimens in an average of 10% of the cases (17% in USA, 8% in Japan, and 0-15% in Europe). Simultaneous dual gastric cancers were found to have an incidence of 13% in a study; hence, pre- and perioperative evaluation should be done in detail. Multicentric lesions are usually more frequent in the 1/3 distal portion of the stomach, more prevalent in men (male/female ratio 7/1), and is more common in bulgy and differentiated types. Sixty percent of cases are histologically differentiated and 40% are undifferentiated. Macroscopically, 20% of the early gastric cancer cases are bulgy and 80% are of sunken type. Superficial types are quite rare. The incidence of macroscopic types of early gastric carcinoma differs from country to country; Type III is the most frequent form in USA while Type IIc (30%) is the most frequent form in Japan. Overall the incidence of lymph node metastasis in early gastric cancer is 7.5-10%.
Lymph node metastasis occurs in 7.5-10% of mucosal carcinomas, perigastric involvement occurs at a rate of 3%, and extra gastric involvement has an incidence of 0.5%. Lymph node metastasis in the submucosal carcinoma has an incidence of 12-19%, perigastric involvement 14%, and extragastric involvement 5.1%.
Exophytic ....................... that protrudes
Flat or sunken ............. that is flat, elevated, or depressed
Dimpled……………. that is excavated
Pyloric antrum=50-60%
Cardia=25%
Corpus/fundus=15%
40% of small curvature, 12% of big curvature
Small curvature localization of the antropyloric area is the most frequent!
Lymph node involvement indicators: a. if lesion is in the mucosa b. if the lesion is smaller than 2 cm c. If it is not Type I, type IIa, type IIb, IIc and contains no ulceration. If it is histologically differentiated, metastasis of the lymph node occurs less.
Tumor cells move to serosa by passing all layers in advanced gastric carcinoma. These types of carcinomas are macroscopically examined in 4 types. (Borrmann classification) (Figure 2):
Type I (polypoid carcinoma): Tumor is in the appearance of a big sessile polyp reaching through the gastric lumen and has ulcer or erosions on it.
Type II (ulcero-carcinoma): Tumor infiltrates deep tissues or is located on sides of the gastric wall, and has ulcers on it.
Type III (ulcero-infiltrative): Tumor is 2-8 cm in diameter, shaped as the edges are upturned and irregular. It has widespread and deep ulcerated invasion in gastric wall and sides of the lesion.
Type IV (diffuse infiltrative carcinoma): Tumor can cover a large part or all of the stomach. Pili of the gastric mucosa might be erased and there might superficial ulcers and bulging type nodes towards the lumen. Gastric wall shows diffuse thickening in the area where the tumor is located and its flexibility is reduced [8].
While the incidence of distal gastric cancer decreases nowadays, proximal gastric tumors continue to increase. Currently, gastric cardia cancers constitute 50% of all gastric adenocarcinomas.
Four macroscopic types are distinguished in advanced gastric cancers. (Borrmann Classification)
The main prognostic factors are of gastric wall thickness, spread to lymph nodes, and presence of distant metastases.
They are 95% adenocarcinomas.
Adeno Ca: It may have a well, intermediate, and less differentiated histological types. Types that show prominent papillary and glandular arrangements are good differentiated. Gradual decrease of these is the sign of the loss of differentiation and implies a poor prognosis.
Gastric cancers are microscopically divided into two: intestinal and diffuse type (Lauren classification). In the intestinal type, the prognosis is better. Diffuse type is cytologically less differentiated, and can contain signet. Cancer in early gastric cancer is limited to the mucosa and submucosa. Early gastric cancer shows a treatable lesion with no symptoms. Tumors that advanced to muscularis mucosa are called advanced gastric cancer [38].
Adeno Ca histological grading: Made according to the degree of differentiation at the gland direction.
It is graded as well differentiated if the ratio of glandular structures in tumor constitutes more than 95% of the tumor mass, moderate differentiated if this figure is between 50-95%, and poor differentiated if it is between 5-49%. Sometimes a tumor may show different differentiation degrees and in this case it should be graded by the most advanced grade.
Intestinal type is characterized with the tendency to form tubular structured gland that malign cells’ mimicking the intestinal glands. These tumors are usually well or intermediate differentiated. This type is associated more with environmental and dietary risk factors in men and has a tendency to be seen in high-incidence areas. They are seen with metaplasia or chronic gastritis. They often occur in elderly patients and show tendency to spread to distant organs hematogenously.
Diffuse type lacks a regular gland occurrence; it is usually poor differentiated and contains several signet ring cell. Diffuse type tumors are more common in women and in younger patients with no history of gastritis; and they spread through transmural or lymphatic ways. It is associated with familial cases and thought to have a genetic etiology. Intraperitoneal metastases are common. Diffuse type appears to be associated with obesity. It is also associated with blood group A [38].
Activation of oncogenes, inactivation of tumor suppressor genes, cellular adhesion reduction are genetic changes associated with adenocarcinoma. When inactivation of P53 and p16 tumor suppressor genes are seen in gastric cancer, adenomatous polyposis coli (APC) gene mutation is seen to a greater extent in the intestinal type. Decrease or loss of the cell adhesion molecule E-cadherin is seen in 50% of the diffuse type.
MUC2; Cardia
MUC5AC; Antrum ca
It is generalized 90% of the CDX2 cases (transformation from gastric phenotype to intestinal phenotype)
80% of Hep Par-1 is positive [50].
MSI is seen in 13-44%,
MSI is positive advanced phase intestinal type cancer.
MUC6, CTNN2, GLI3; RNF43; in diffuse type 14%, intestinal 0%
TGF Beta=Diffuse type, tumorigenesis
Up regulation of many growth factors has been shown in gastric carcinomas. It is known that c-erbB2/neu (HER2) is an important indicator for prognosis and treatment [54].
EGFR2 (HER2, ERBB2)
Amplification at the rate of 7-34% or over expression 15% in total, 25% in intestinal type.
Positive HER Prognosis? [55]
HER2 (c-erbB2/neu)-(Human epidermal growth factor receptor 2)
A transmembrane receptor tyrosine kinase from the EGFR family.
There is not a specifically known ligand.
HER2/neu; Intensity of immunohistochemical staining (Figure 6)
2-3 (4) µm incision
Formalin-fixed, paraffin-embedded tissue blocks
Appropriate fixation time: 12-24 hours
“Fluorescent/chromogene”
Hybridization of the labeled probe into the specific gene area is of Her2/neu on the surface of 17th chromosome.
Excessive involvement of C-erb B2 indicates a poor prognosis.
Ras gene expression is mostly in the intestinal type.
C-myc is associated with the invasion and peritoneal spread.
CEA, CA19-9, DNA ploidy assay, and cell proliferation index determine the prognosis
p53 height is a sign of poor prognosis [56].
Lymph node spread with tumor penetration: As tumor depth increases, more lymph node is affected from metastasis:
Despite advanced diagnostic procedures, no important progress has been made yet in the early diagnosis of gastric cancers.
The only chance for detecting early-stage cases is to use endoscopic methods in an appropriate and timely fashion. Therefore, individuals older than middle age who present with dyspeptic complaints unresponsive to medical treatment, anemia, and significant weight loss, endoscopy should be performed without delay. When an ulcer is seen in the stomach during endoscopic examination, at least 7-8 biopsies should be taken from around the ulcer.
Routine biochemical tests studied in early gastric cancer may be normal. Biochemical tests might also be normal in advanced patients, except for iron deficiency anemia that is usually observed due to blood loss. Depending on the liver involvement deterioration in liver enzymes can be seen. In 20-60% of cases CEA, in 25-50% of cases CA19.9, and in 35% of cases CA72.4 are positive. Tumor associated antigen (2H6-antigen) is found in an extremely high percentage (65%) of the early-stage gastric cancer (I and II). The corresponding rate for CA19.9 is 25%. The level of 2H6 gradually decreases by 12th week postoperatively. The combination of the CA19.9 + 2H6 antigen may be a useful marker for early diagnosis.
Endoscopic and radiological diagnostic methods: Especially with the development of flexible endoscopes, there has been a significant increase in early diagnosis of gastric cancers. Diagnosis rate in multiple biopsies is above 95% [58]. Cell sampling for cytologic examination can also be done with endoscopic brushes. Staining techniques (chromoscopy) can be applied in order to help endoscopy to diagnose early gastric cancers.
Double-contrast barium graphies are valuable for diagnosis but this method should be supported with endoscopy in order to provide diagnosis. Especially, digital radiography’s sensitivity is around 75%; it has specificity around 90%.
Transabdominal ultrasonography can detect tumor and lymph nodes with an accuracy of 40% to 65%.
Endoscopic ultrasonography is a diagnostic method that was developed in recent years and has been spreading increasingly. It is useful in detecting early gastric cancers’ regional lymph involvement and the depth of tumor infiltration. Endoscopic ultrasonography can detect the mural invasion by tumor with an accuracy of 80%; and T1 and T2 tumors can be distinguished from each other with an accuracy of 90-99%.
Various methods including computed tomography, spiral tomography, and arteriography are used for tumor staging and prognostication. While computed tomography has a sensitivity of 65-90% in advanced cancers, it decreases to 50% in early cancers. Computed tomography can also detect the tumor stage 60-70%, lymph node stage 40-70%, peritoneal nodes 70% and liver metastases with accuracies of 60% respectively. Magnetic resonance imaging has similar figures of accuracy.
Factors affecting survival in surgically resectable gastric cancer are the degree of tumor penetration (wall depth), lymph node involvement, and distant metastasis. TNM staging system is used in gastric cancer to divide it into 4 stages [59].
STAGE I: The tumor may be limited to mucosa, submucosa, muscularis, or serosa. There is no regional lymph node involvement. T1 N0 M0 T2 N0 M0 T3 N0 M0.
STAGE II: Gastric wall is widely involved or there is metastasis to perigastric lymph nodes along with gastric wall involvement at any level. T4 N0 M0, Tx N1, M0.
STAGE III: Together with the gastric wall involvement at any layer, lymph nodes that are 3 cm further from the primary tumor are also involved. Tx N2 M0.
STAGE IV: There are distant metastases. Tx Nx M 1
TNM classification
Stage of the disease, condition of lymph nodes, and penetration degree of the tumor tissue in the gastric wall are important factors that determine the prognosis [60].
Although prognosis is very good at early stages, 60% of cases lost the chance of radical surgery when they are diagnosed. Cancer in most of these patients is either at stage III or stage IV. The number of metastasized lymph nodes and the degree of the serosal invasion affect prognosis in an adverse manner. Five-year survival is 90% at stage IA, 80% at stage IB, 65% at stage II, 50% at stage IIIA, 30% at stage IIIB and 5% at stage IV [61].
Prognosis in intestinal type tumors is better compared to the diffuse type [62]. DNA ploidy condition of tumor cells also affects prognosis, with the prognosis of aneuploidic tumors being worse. Also, it is reported that certain molecules such as glucose transport protein-1 alpha synthesized by cancer cells, annexins, and soluble e-cadherin can be used for prognosis evaluation. Prognosis is better in gastric cancer that is weakly stained for CEA. It is debated whether the prognosis of HLA DR4 (+) cases is good or not.
Factors affecting prognosis in early gastric cancer are; invasion depth (mucosal, submucosal spread), lymph node metastasis, and lesion size.
Five-year survival is 87% when lymph node involvement is negative; 15-25% if there exist intramucosal spread+lymph node involvement; and 3-5% if there exist submucosal spread+lymph node involvement. Fiveyear survival after surgery is 90-95% in cases with intramucosal spread; 86% with submucosal spread, and 66% with submucosal spread+lymph node involvement. When it comes to ten-year survival, it is 58-87% when lymph node involvement is positive and 82-97% when negative [57].
Gastric lymphomas: They constitute less than 10% of gastric neoplasias [63]. Non-Hodgkin lymphoma is most commonly seen in stomach except for lymph nodes. Most frequent lymphoma type is the diffuse histiocytic lymphoma. Symptoms are similar to those in adenocarcinomas.
Macroscopic appearance might be polypoid, ulcerative, or infiltrative. Diagnosis is via endoscopic biopsy. Surgery can be applied to approximately 95% of patients at early stage. Curative resection can be done to more than 80% of these cases. Five-year survival is around 80-95% at stage IE, 40- 75% at stage IIE, 10-30% at stage IIIE, and 5-8% at stage IV. In cases where surgery cannot be applied, radiotherapy should be suggested. Surgery, chemotherapy, and radiotherapy are tried in advanced stages.
MALT-type lymphoma is known to have a close relationship with H.pylori. Some of these patients are being cured with H.pylori eradication treatment. However, effectiveness of the treatment is not definitive in the long term. Radiotherapy is also effective in MALT lymphomas [64].
Gastric sarcomas: It covers 13% of malignant tumors of the stomach. The most common type is leiomyosarcoma, whereas angiosarcoma, fibrosarcoma, liposarcoma are seen less frequently. Sarcomas are usually seen in people above 50 years as the adenocarcinomas; and the first symptom might be heavy bleeding. Wide resection is recommended. Fiveyear survival rate is around 35-50% in cases where resection is made.
Metastatic gastric tumors: Primarily, a patient with a non-gastric malignancy should be examined when the patient comes with symptoms related to upper gastrointestinal system such as nausea, vomiting, pain, or bleeding. Metastasis of malignant melanoma is seen as the most frequent from of metastatic gastric tumors. Lung, liver, ovary, testicle, colon cancer metastases can also be encountered, as well as gastric metastases of kaposi sarcoma in people with AIDS [65,66].
Gastric carcinoids: These tumors are very rare; theyoriginate from enterochromaffin cells in the gastrointestinal system. Three percent of all gastric tumors are located in the stomach. Symptoms like hot flushes in face, tachycardia, diarrhea, pain and nausea might occur due to the secretion of serotonin and histamine. All carcinoids are potentially malignant and may metastasize to distant sites. Tumors smaller than 2 cm can be removed endoscopically or with limited surgical excision. A wide surgery is needed for larger lesions. Chemotherapy should be tried for patients that are not suitable for surgery and have distant metastases. Five-year survival is around 50% [67].
Gastric polyps: These are rarely seen. They are adenomatous or hyperplastic epithelial lesions. The risk of becoming cancerous is especially high for villous adenomas. They may cause nonspecific dyspeptic complaints; and they may present with bleeding or obstructive symptoms. Most of the patients with gastric polyps have achlorhydria. Incidence of atrophic gastritis, pernicious anemia, and gastric cancer is increased in these cases. Gastric polyps are seen in polyposis syndromes such as Gardner, Peutz-Jeghers, and Cronkhite-Canada syndromes.
Pancreatic remains: They are rare and characterized with the ectopic pancreas tissue in the stomach. They might present themselves as bleeding, obstruction, or pancreatitis. Their treatment is surgical excision.
Metastatic gastric cancer: Gastric metastases of other organ carcinomas are rare. Malign tumors that most frequently make metastasis are; breast cancer, malignant melanoma, lymphoma, and leukemia.
Other histologic types: Neuroendocrine tumors are histologically divided into 3 types including well differentiated (argyrophil cell tumors, enterochromaffin-like, and G cell), poorly differentiated (neuroendocrine carcinomas), and mixed type (adeno neuroendocrine carcinomas). Although it is rare, tumors with different cell types can be seen in the stomach such as parietal cell adenocarcinoma, hepatoid adenocarcinoma, choriocarcinoma, and lymphoepithelioma-like carcinoma [63].
Patients are mostly asymptomatic in the early stage. Symptoms frequently arise from the full involvement of gastric wall or prevention of food flow in the stomach by the tumor. They may also arise after adjacent organ invasion. There is an average of 6-9 month period between appearance of clinical symptoms and diagnosis. Most frequent symptoms of gastric cancers are anorexia, weight loss, persistent indigestion, and asthenia. Also, there might be heartburn, intumescence, nausea, and vomiting. Pain can sometimes alleviate with food intake or antacids. A pain resembling angina pectoris might be seen in cardia cancer. Besides, dysphagia and vomiting are seen frequently in tumors of this area. Gastric cancer usually causes anemia as a result of occult bleeding. Hematemesis or melena are only seen in 5% of cases. There is palpable mass in the gastric in half of the gastric cancer patients. 10% of patients present with metastasic signs such as sacites, anemia, and pleural fluid. Palpable mass with rectal and vaginal examination shows Blumer shelf or Krukenberg tumor.
The Five-year survival rate in patients with gastric adenocarcinoma is on average 10-15%.This figure goes higher in early gastric cancer but these cases which can be operated constitute only 5-16% of all cases. Surgery cannot be applied to approximately 15% of diagnosed patients due to the early stage of the disease or the risk of surgery. Curative resection can be made to only 85% of remaining patients. Palliative surgery, bypass surgery or only biopsy can be performed to remaining patients. Endoscopic mucosal resection in the treatment of early gastric cancer has been becoming more widespread in recent years and its prognosis is very good. Adjuvant chemotherapy is beneficial for reducing both systemic and local recurrence in patients especially with lymph node involvement after surgery. In advanced gastric patients that do not have any chance of curative surgery, various multiple chemotherapy regimens (mitomycin C, 5-FU, cisplatin and etoposide, doxoruicin, cisplatin regimens) are tried. Studies support early postoperative chemotherapy application. Neoadjuvant chemotherapy’s contribution on mortality and morbidity could not been shown yet. The role of radiotherapy in the treatment of gastric cancer is very limited. It can provide an increase in the palliation of symptoms and survival when it is used along with chemotherapy in locally advanced cases. Surrounding tissues cannot tolerate radiation so much because of the localization of the stomach. Therefore, limited use with other treatment options might be an option in advanced stages. Surgical treatment of early gastric cancer had been done until 20 years ago. Nowadays, however, endoscopic mucosectomy (submucosal resection) has replaced it. Thus, it is understood that lesions are curatively treated without proceeding to surgery. Mucosectomy is indicated if tumor is smaller than 2 cm, superficial, and well differentiated. It should be carefully examined with endoscopic ultrasonography before the endoscopy to ensure that these lesions are not overflown outside submucosa [57].
Download Provisional PDF Here
Article Type: Review Article
Citation: Kayacetin S (2016) Approach to Gastric Cancers. J Gastric Disord Ther 2(2): doi http://dx.doi. org/10.16966/2381-8689.119
Copyright: © 2016 Kayacetin S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access