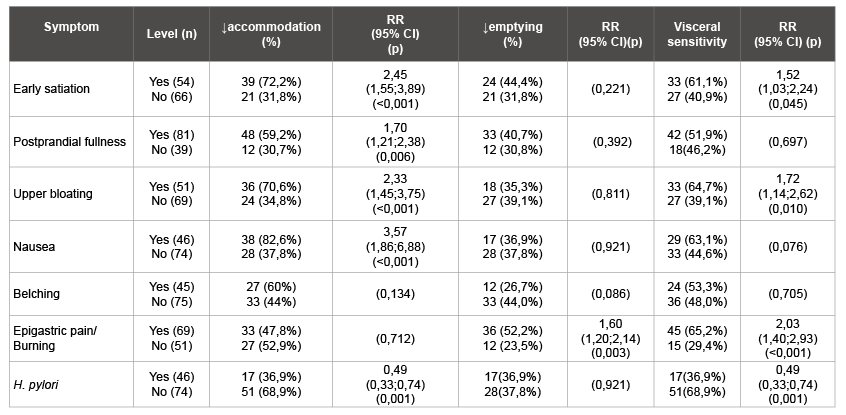
Table 1: Dyspepsia symptoms and results of WDUCT (RR, 95% CI)
Andrey E Dorofeyev Vyacheslav G Perederiy Tatyana E Kugler*
Bogomolets National Medical University, Kiev, Ukraine*Corresponding author: Tatyana Kugler, 01053, Shevchenko bul., 17, Kiev, Ukraine, Tel: +38 (044) 235-62-35, +38 050 1414080; Fax: +38 (044) 234-92-76; E-mail: kugler2@mail.ru
The major pathophysiological causes of Functional Dyspepsia (FD) are motility dysfunction and visceral hypersensitivity. APUD system plays a role in the regulation of gastric motility and acid secretion. But we have no quite convenient way to study gut functions. That’s why we cannot interpreter laboratory and instrumental examination according to dyspepsia manifestations. The aim of this study was to examine the relationship between ghrelin, Glucagon-Like Peptid-1 (GLP-1) and clinical features of FD.
A total of 125 patients with FD according to the Rome III criteria and control group of 30 healthy volunteers were included in this cross-sectional study. FD group were carried out by means of clinical, laboratory and instrumental examination. Acid secretion was investigated by gastric pH monitoring. Measuring the mean cross-sectional area of the fundus was performed to investigate the accommodation and gastric emptying during and after water intake. During the test, abdominal symptoms were evaluated using the 4-point Likert scale. Blood samples were taken for acyl ghrelin and GLP-1 by ELISA method.
The Water-Drinking Ultrasonography Combined Test (WDUCT) revealed the impairment of gastric accommodation in FD, delayed emptying and statistically significant hyperesthesia in the FD group compared with healthy controls (p<0.05). PDS and overlap FD were independently associated with gut motor disturbances instead of EBS (p<0.01). Ghrelin and GLP-1 levels were correlated well with the results of WDUCT. The plasma ghrelin levels were significantly lower in FD patients, especially in PDS compared with control (p<0.05). The plasma GLP-1 levels were higher in FD patients (p<0.01). Ghrelin levels correlate with GLP-1 levels (R=0,492, p<0,001).
PDS and overlap FD are associated with decreased fasting ghrelin concentrations. These data suggest a possible role for acyl ghrelin physiology in the pathogenesis of gastric dysmotility. Results of research revealed potential endpoints for future clinical trials of FD.
Accommodation; Gastric emptying; Visceral hypersensitivity; Water-drinking test; Early satiation; Ghrelin; Glucagon-like peptid-1
Functional Gastrointestinal (GI) disorders are the actual problem of modern gastroenterology. Syndrome known as dyspepsia occurs in the same structural and functional pathology. According to research Suzuki et al. upper gastrointestinal endoscopy findings appear normal in approximately 75% of patients with dyspepsia and most of these individuals are diagnosed with Functional Dyspepsia (FD) [1]. FD is extremely common, affecting up to 15–20% of the general population and is associated with markedly decreased quality of life and substantial health-care costs [2]. Understanding the particular qualities of the FD patients provides important information concerning the various factors affecting consultation choice, in turn to lead to clarify the etiology of FD patients [3].
Functional dyspepsia is determined as the presence of dyspeptic symptoms in the absence of any organic cause that can explains them. The Rome III consensus divides FD into Postprandial Distress Syndrome (PDS) and Epigastric Pain Syndrome (EPS). Epidemiological studies in the USA and Europe confirmed the presence of both subgroups, with great
overlap between EPS and PDS [4]. The available treatment options for FD are of limited effectiveness, which reflects its poorly understood pathogenesis. Studies indicate that FD is a heterogeneous disorder, in which different pathophysiological mechanisms underlie specific symptom patterns [5]. Traditionally, gastric abnormalities (such as impaired accommodation, delayed emptying and hypersensitivity) have believed to be involved in the pathophysiology of FD [2]. Tests of motility and sensory gastric functions are available in health service, but there is no “gold standard”. Gastric barostat is not widely used because the procedure is extremely invasive [6]. The results of electrogastrography are depending on the location of electrodes, acid secretion or duodenal reflux. Imaging methods such as X-ray and scintigraphy with 99Tc or 111In cannot be extensively used because of radiation exposure and long examination time. Video capsule endoscopy is a very expensive method and not applicable in routine practice. 13C-urea breath tests for evaluation the gastric emptying are available only in specialized clinics. On the other hand, ultrasonography does not require radiation, special chemical substances or unusual diagnostic equipment. In terms of money and timesaving, Water-Drinking Ultrasonography Combined Test (WDUCT) is useful for examination of FD patients.
Gastrointestinal hormones such as cholecystokinin, gastric inhibitor peptide, Glucagon-Like Peptide-1 (GLP-1), motilin and ghrelin regulate gastric functions. Previous publications reported that motilin and ghrelin stimulated gut motility and acid secretion but GLP-1inhibited it [7]. But results were controversial and regulation of ghrelin and GLP-1 secretion need to be clarified. As ghrelin and GLP-1 have influence on gastric functions, these peptides may play a pathophysiological role in FD. Therefore, we made this study with purpose to compare gut motor and sensory function between FD patients and healthy controls and explore the roles of different APUD- peptides in this motility effect.
This is a cross-sectional study conducted in the Department of Internal medicine, Central Clinical Hospital №3, Donetsk, Ukraine, between November 2013 and November 2014. The study protocol was approved by the Ethics committee of the Central Clinical Hospital #3 on 11 November 2013 (№169), and carried out in accordance with the Declaration of Helsinki.
This study included 125 patients with a diagnosis of FD. According to the Rome III criteria patients were divided into three groups. We used Russian language-validated questionnaires. Subjects will be eligible to enter the study if all of the following criteria are met: male or female subjects aged 18-75 years; Rome III Criteria must be fulfilled for the 3 months prior to informed consent with symptom onset at least 6 months prior to informed consent; normal endoscopy result within the 6 months; female subjects of childbearing potential must provide a negative pregnancy test. Exclusion criteria included: subjects taking drugs that affect gut motility, gut sensitivity and/or acid secretion; non-steroidal antiinflammatory drugs; organic gastrointestinal disease; history of surgery that can affect gastrointestinal motility including endoscopic surgery for Gastro-Esophageal Reflux Disease (GERD) and obesity; irritable bowel syndrome; chronic idiopathic nausea; Type I or Type II diabetes; active uncontrolled psychiatric and/or psychosomatic disorders; BMI over 30 kg/m2 ; clinically significant renal, hepatic, cardiovascular, pulmonary, endocrine, metabolic, or hematological condition. Healthy subjects did not have dyspepsia symptoms or drug allergy. All subjects recruited in the study were subjected to upper GI endoscopy, biochemistry analyses and abdominal ultrasound to exclude organic alimentary tract diseases. The written informed consent was achieved from all study participants.
During the water-drinking period, subjects ingested 200 ml of water at 3-min intervals 5 times (total 1000 ml) by ingesting water through a straw. The test was discontinued if they felt some discomfort. Examination of the gastric emptying was conducted 5 and 10 min after the completion of drinking 1000 ml (or discontinuation). Measuring the mean cross-sectional area of the fundus during and after water intake was performed to investigate the fundic accommodation and gastric emptying. All ultrasonography examinations were performed using an Ultima PA (1-5 MHz convex-type probe). The patient was asked to grade the intensity (0, absent; 1, mild; 2, relevant; and 3, severe) of the pain during water intake. The normal range of cross-sectional area of the fornix was set using dates of 30 healthy volunteers. Cases outside the normal range were diagnosed with a motor or sensory disorder.
Venous blood was sampled from 8.00 to 10.00 a.m. for measurement of plasma peptides. All persons were assumed to be fasting after 12 hours. Plasma was separated and samples stored at -70°C for subsequent analysis of GLP-1 and ghrelin concentrations, using ELISA. Ghrelin was measured by a commercial ELISA kit (RayBio Human, USA); intra- and inter assay Coefficients of Variation (CV) were <10% and <15%, respectively; the minimum detectable concentration of Ghrelin is 161 pg/ml or 12.46 pM. Detection range: 0.1-1,000 pg/ml.GLP-1 were also measured by a commercial ELISA kit (YK160, Japan); intra- and inter assay coefficients of variation (CV) were 4, 69-10, 67% and 9, 63-17, 57%, respectively. Detection range: 0,206-50 ng / ml.
Data were evaluated with Medstat (MS 000020, Ukraine) and NCSS (PASS 11) (SN 2645878484), which are commercially available statistics software package. All values were expressed as the mean ± standard deviation. Student’s and Wilcoxon’s tests were performed for evaluation of continuous variables and chi square test for frequency variables. P values of less than 0.05 were considered to indicate statistical significance. Relative Risk (RR) with 95% Confidence Interval (CI) was computed. Biochemical results displayed normal distribution.
The first group consisted of 60 (48.0 %) patients with PDS, the second one -44 (35.2%) patients with overlapping EPS and PDS, and the third one - 21 (16.8 %) patients with EPS. The age limits of the patients were 18-67 years (mean age 44, 07 ± 15,54years). Study group consisted of 37 men (29.8%) and 88 women (70.2%). The mean body mass index (BMI) of group was 22.8 ± 4.3 kg/m2 . The healthy control group consisted of 30 subjects (7 males and 23 females) with a mean age of 39.2 ± 14.8 (18-59) years and a mean BMI of 23.3 ± 5.37 kg/m2 . There was a not significant statistical difference between two groups in the distribution of sex, age, BMI, height and weight.
We developed a novel WDUCT to assess gastric motility and sensory functions of FD patients compared with healthy controls (Table 1). First we studied gastric accommodation (Figure 1), emptying and sensation. The mean cross-sectional area of the fornix before water intake in the control group was 9.67 ± 1.72 cm2 , in FD group – 11.49 ± 1.61 cm2 , p=0.61. After 200 ml-14.12 ± 2.95 cm2 vs. 15.11 ± 2.72 cm2 , p=0.729. After 400 ml–18.87 ± 4.12 cm2 vs. 19.64 ± 2.03 cm2 , p=0.088. After 600 ml–24.05 ± 5.02 cm2 vs. 25.29 ± 3.2 cm2 , p=0.067. After 800 ml–30.71 ± 7.19 cm2 vs. 29.59 ± 4.11 cm2 , p=0.07. The mean cross-sectional area of the fornix after 1000 ml of water intake was significantly lower in the FD group–33.93 ± 4.03 cm2 compared with the control group–37.02 ± 6.22 cm2 , p=0.022, suggesting the impairment of gastric accommodation in FD.
We have also experienced delayed emptying in the FD group (Figure 2). The percentages of the cross-sectional area of the fornix 5 min after water drinking was 94, 81 ± 11, 11% in the FD group and 80, 43 ± 7, 14% in the control, p =0.011. The percentages of the cross-sectional area of the fornix after 10 min was 69, 42 ± 8, 83%, and in control group–76, 21 ± 12, 72%, p=0.073. This implies that the survey can reduce and evaluate only after 5 minutes in the future because of statistical significant differences. The mean value of the cross-sectional area of the fornix in the FD group was higher than that in the control group, suggesting delayed emptying in FD.
In the FD group, such symptoms as abdominal fullness and epigastric pain developed immediately after the initiation of water intake, and the Likert scale ended to be high compared with the controls (Figure 3). The VAS score differed significantly after 400 ml between the control and FD groups (after 200 ml of water 0.18 ± 0.13 vs. 0.01 ± 0.41, p=0.39; after 400 ml 0.41 ± 0.23 vs. 0.13 ± 0.31, p=0.047; after 600 ml 0.83 ± 1.02 vs. 0.2 ± 0.32, p = 0.03; after 800 ml 1.01 ± 1.02 vs. 0.25 ± 0.32, p=0.002, after 1000 ml 1.35 ± 1.04 vs. 0.3 ± 0.48, p<0.001), suggesting hyperesthesia in the FD group.

Table 1: Dyspepsia symptoms and results of WDUCT (RR, 95% CI)
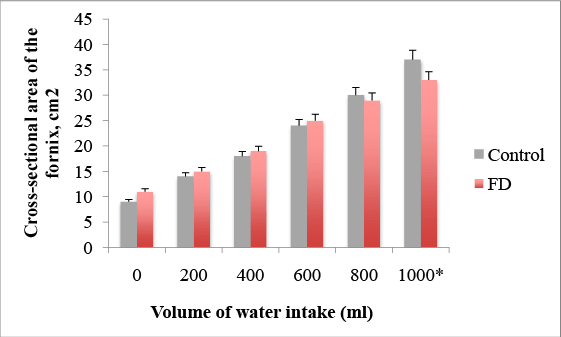
Figure 1: Impairment of gastric accommodation after 1000 ml of water intake in FD patients, *p=0.022
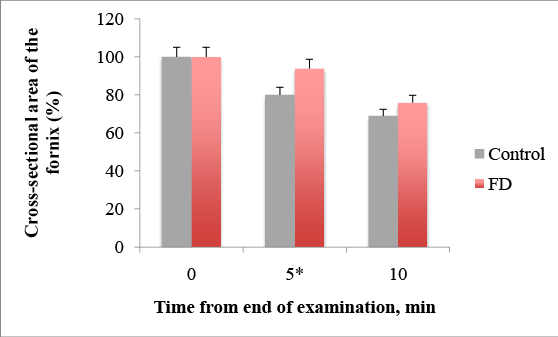
Figure 2: Delayed emptying after 5 min from end of water-intake in FD patients, * p= 0.011
A comparison of the prevalence of individual symptoms in those with normal and reduced accommodation, normal and delayed gastric emptying, normal and hypersensitivity were summarized in (Table 1). This study has revealed connections between dyspeptic symptoms and gut motor function. The impairment of accommodation was found in 50.0% of FD patients and was typical for patients with postprandial symptoms, p<0,001 (Figure 4). Delayed gastric emptying was typical for 37.5% FD patients and 52.2% patients with epigastric pain. This type has hyperacid pH-gram frequently. It is assumed that acidification of the duodenum promotes such mechanisms as impaired gastric accommodation, delayed gastric emptying and hypersensitivity. Visceral hypersensitivity occurred the half of the FD group and prevailed among patients with early satiety (p=0,045), bloating (p=0,010) and pain (p<0,001). The disturbances of gastric motor-evacuation function were dependent on the clinical subtype of FD. Impairment of the accommodation and / or emptying were encountered more frequently in the PDS and overlap group than in the EBS, p<0,05. Visceral hypersensitivity was prevailed in the overlap group, p<0,001.
The plasma level of acyl ghrelin was significantly lower in FD patients than in the control group (Figure 5): 461, 6 ± 283, 4 pg / ml and 528,1 ± 152,4 pg / ml, respectively (p=0.033). When stratified according to the subtype of dyspepsia, the plasma levels of acyl ghrelin were significantly lower in the PDS than in the control group (p=0,041). In the EPS, the plasma levels of acyl ghrelin were significantly higher than those in the PDS and overlap group (p<0, 01).The plasma level of GLP-1 was significantly higher in FD patients than in the control group (Figure 6): 3, 5 ± 2, 3ng/ ml vs. 2, 5 ± 0, 3 pg/ml (p<0,01). There were no significant differences in the plasma GLP-1 levels between the different sub types of FD group. It was also a significant linear correlation (Figure 7) between the plasma levels of GLP-1 and the plasma levels of acyl ghrelin (R=0,492, P<0.001). As contributing factors of the ghrelin and GLP-1 secretion in patients with FD were studied gender, age, BMI, disease duration, WDUCT and pH monitoring results, subtype of FD and infection H. pylori (Table 2).
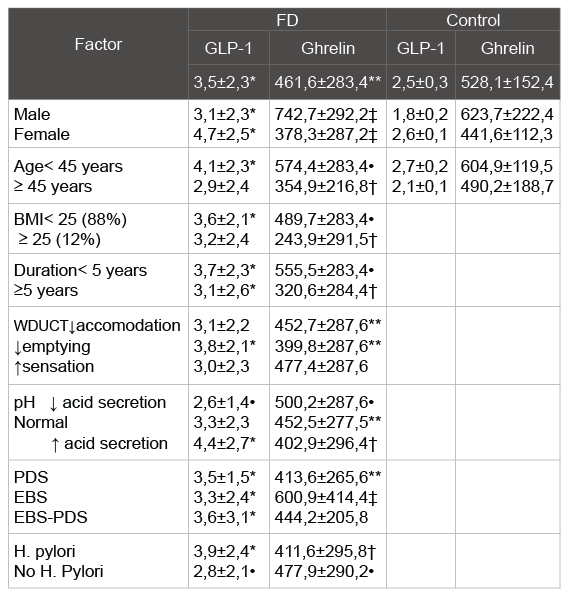
Table 2: Plasmaacyl ghrelin and GLP-1 levels in the FD group and the
study control group
*- p<0, 01, **- p<0, 05 as compared with the value in the control group
†-p<0, 05 as compared with the value in the control group and inside the
group
‡-p<0, 01, • - p<0, 05 as compared with the value inside the group
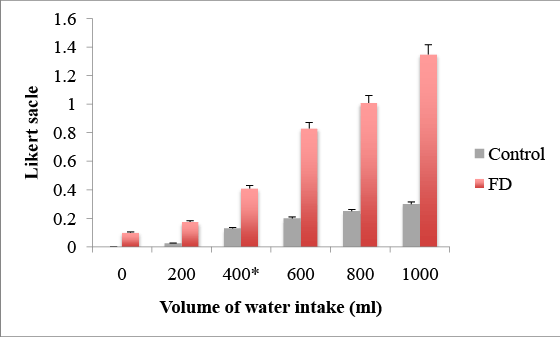
Figure 3: Visceral hypersensitivity after 400ml of water intake in FD patients, * p=0.047
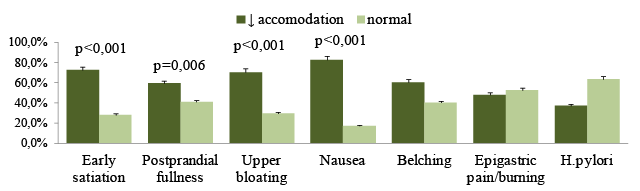
Figure 4: Prevalence of dyspepsia symptoms and H. pylori (%) in patients diagnosed with normal and impairment of gastric accommodation
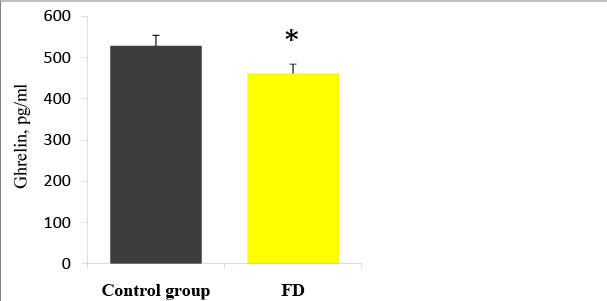
Figure 5: Plasma ghrelin levels in FD patients and the control group, *p<0.05
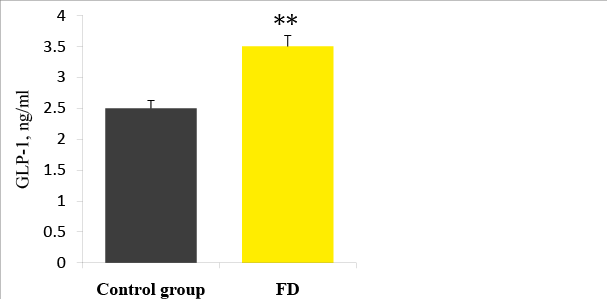
Figure 6: Plasma GLP-1 levels in FD patients and the control group. ** p<0.01
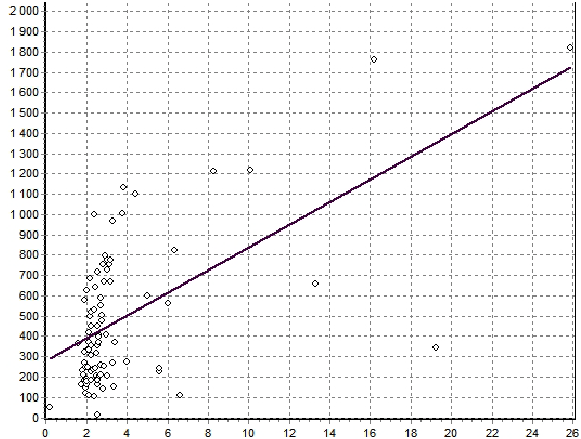
Figure 7: Correlation between the plasma levels of ghrelin and GLP-1 in FD patients (R=0,492, p<0,001).
The pathophysiological mechanisms of FD include GI dysmotility, perception disorders, acid hypersensitivity, psychological factors and duodenal dysfunction. The origin of impaired accommodation in FD is unknown, but conceptually it can be caused by abnormalities of the vago vagal reflex, the intrinsic inhibitory innervation (myenteric plexus) or the smooth muscles of the proximal stomach [2].Visceral hypersensitivity to gastric distension is detected in 34-65% of patients with FD and correlates with severity of dyspeptic symptoms. Van Oudenhove et al. first found a significant and independent influence of gastric sensitivity and abuse history on gastric sensation scores in FD [8].
The WDUCT revealed abnormalities in gastric motility and sensation in patients with FD compared with healthy controls. But most diagnostic tests of the gastric motility characterize only gastric emptying. We were interested to research relation between accommodation and postprandial symptoms. This study demonstrated that impaired gastric accommodation causes early satiety, postprandial fullness, bloating and nausea, but not delayed gastric emptying. The WDUCT can be readily performed, well tolerated by the patient and widely used in routine clinical practice. Promotion of gastric accommodation may be an effective therapy for patients with FD who report early satiety and postprandial fullness.
The changing serum ghrelin level in patients with FD is still a controversial topic. While total serum ghrelin is reported to be higher in patients with FD in some studies, other studies show lower levels of serum ghrelin, as compared to healthy control. Furthermore, the pathogenic role of these alterations is still unclear [9]. It was the first study to claim a potential association between plasma ghrelin and GLP-1 levels and gastric accommodation, emptying and sensation. We confirmed by means of the results of WDUCT and pH monitoring that ghrelin stimulates upper gastrointestinal motility and acid secretion, but effects of GLP- 1 are controversial. The present study has shown for the first time that the plasma level of ghrelin correlates with plasma level of GLP-1. As a possible explanation for the high level of GLP-1 in FD patients, it has been suggested that compensatory secretion of ghrelin is enhanced in FD patients in order to normalize the impaired gut motility. Increasing GLP-1 in response to increased ghrelin and vice versa may indicate controversial work in the maintenance of the equilibrium state of the gastrointestinal tract. H.pylori infection is reported to be associated with lower plasma ghrelin levels, both in PDS and EBS. The validity of this study was limited because of some positions. It was impossible to follow the changes in the secretion of GLP-1 and ghrelin in the development of FD because of crosssectional design of this study. Determination of ghrelin and GLP-1 plasma levels was investigated as potential biomarkers of FD. But the controls group small sample size (n=30) is not representative for population to assess the normal assay of ghrelin and GLP-1 as diagnostic criteria of FD.
The results of the present study suggest that the WDUCT come to be used for diagnosis gastric motor and sensation dysfunction, particularly in PDS and EBS-PDS patients. This study has also shown the effects of ghrelin and GLP-1 on the pathogenesis of FD, indicating the presence of complex interactions in the regulation of brain-gut axis. Based on the results of the present study, large-scale neurophysiological trials for examining plasma ghrelin and GLP-1 levels in FD patients should be conducted to find causes of the positive correlation in spite of the different effects of two key gastrointestinal peptides.
Funding: Authors do not have any financial support, any editorial assistance to the underlying research project and/or the preparation of the article for submission.
Conflict of interest: Authors have no conflicts of interest related to the manuscript.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Dorofeyev AE, Perederiy VG, Kugler TE (2015) Do APUD Peptides Play a Role in the Pathogenesis of functional Dyspepsia? J Gastric Disord Ther 1 (1): doi http://dx.doi. org/10.16966/2381-8689.103
Copyright: © 2015 Dorofeyev AE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access