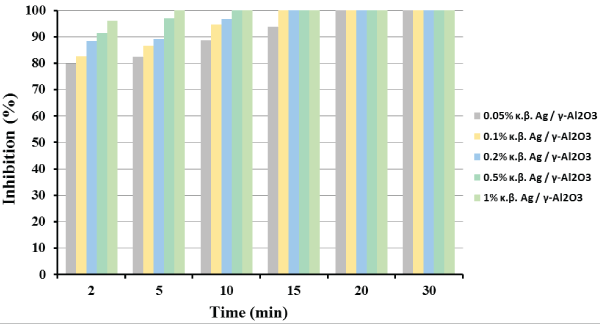
Figure 1: E. coli bacterial inhibition profile as a function of Ag metal loading and reaction time. Reaction conditions: mcat =4.0 g; P=1.3 atm; T=25°C; Stirring Rate=150 rpm.


Theologides CP Theofilou SP Savva PG Olympiou GG Costa CN*
Department of Environmental Management and Technology, Laboratory of Environmental Catalysis, Cyprus University of Technology, P.O. Box: 50329, CY 3606 Limassol, Cyprus*Corresponding author: Costa CN, Department of Environmental Management and Technology, Laboratory of Environmental Catalysis, Cyprus University of Technology, P.O. Box: 50329, CY 3606 Limassol, Cyprus, E-mail: costas.costa@cut.ac.cy
The present paper introduces the new concept of antimicrobial catalysis towards the disinfection/treatment of ships ballast water (SBW), which is considered as a priority issue for the shipping industry. Taking into account the well-known antimicrobial properties of ionic silver (Ag+), five silver supported catalysts (Ag/γ-Al2O3) with various loadings (0.05 wt%, 0.1 wt%, 0.2 wt%, 0.5 wt%, and 1 wt%) were prepared and examined for the antimicrobial treatment of ships ballast water. The bactericidal activity of the aforementioned catalysts was investigated towards the inhibition of E. coli and E. faecalis bacteria. Catalytic experiments were conducted in a three-phase continuous flow stirred tank reactor, used in a semibatch mode. It was found that using the catalyst with the lowest metal loading, the inhibition of E. coli reached 95.8% after 90 min of treatment of an E. coli bacteria containing solution, while the inhibition obtained for E. faecalis was 76.2% after 60 min of treatment of an E. faecalis bacterial solution. The results of the present work indicate that the prepared monometallic catalysts exert their antimicrobial activity within a short period of time, revealing, for the first time ever, that the field of antimicrobial heterogeneous catalysis using deposited ionic silver on a solid support may prove decisive for the disinfection of SBW.
IMO; Water disinfection; Catalysis; Ballast water; Silver
It is widely known [1-6] that ballast water (BW) used by ships, aims to ease sailing conditions by ensuring hull integrity, stability and maneuverability and by compensating cargo’s stevedoring along with ship’s fuel and water consumption. According to statistical data, mainly because of the fact that shipping is the dominant sector of trade [7,6], about 10 billion tons of water are transferred on account of ships ballast tanks [8], which is discharged in various ports across the globe [7,9]. Therefore, the biota contained in ballast water which consists of micro-algae, lots of small invertebrates, larvae of aquatic plants and fish, seeds, spores, eggs, viruses and bacteria [10,4], may survive in ballast tanks and subsequently disrupt the aquatic or non-aquatic ecosystems where they are discharged, being non-indigenous species (1,9-11). As a result, the natural barriers between different marine species are currently to be collapse [12]. Thus, through the essential use of ballast water arises a considerable environmental issue with devastating consequences for both the world economy and public health [1,4-6,7, 9,12-14]. Concerning the latter affected sector, a lot of microorganisms such as bacterial pathogens which are introduced to new marine environments, are held to be responsible for the spread of serious diseases and pandemics in numerous regions of the world [15,11,10,14]. In addition, the high density of those bacteria in ballast water [12,16,10], combined with their extremely rapid reproduction rates, their broad resistance to a wide range of environmental conditions and their ability to survive under harsh conditions, urged the international community to recognize the treatment of ballast’s water bacteria and viruses as a priority issue, in compare with the invasive phytoplankton and zooplankton species [12].
Hence, taking in mind that transportation of pathogenic species by ships, is one of the current issues the shipping industry is trying to address [15,12,10,4,5,17-19], strict regulations are enforced recently by the International Marine Organization (IMO) regarding Human Pathogens: Vibrio cholera (1 cfu/100 ml), E. coli (250 cfu/100 ml) and the bacterial group Intestinal Enterococci (100 cfu/100 ml) [17,15,5,6]. These regulations along with other ballast water management guidelines are included in the International Convention for the Control and Management of Ship’s Ballast Water and Sediments (BWM Convention) which was enacted by IMO on 13 February 2004 and it constitutes the culmination of an international effort for preventing the transfer of non-native species [1,5].
Furthermore, as illustrated in Table 1, following the accomplishment of a brief evaluation, it becomes easy to obtain that the current methods for ballast water treatment are neither appropriate nor effective for the microbial treatment of this kind of water [17,4,6,8]. Therefore, the invention and application of more efficient, affordable and environmentally friendly techniques especially for ballast water microbial treatment, is of major concern as the aforementioned convention is closer than ever before to enter into force, recording the ratification of 50 States which represent the 34,81% of the required 35% of world merchant shipping tonnage [5].
| TREATMENT METHOD | Eco- friendly |
Economical | Sterilization Efficient |
| BW Exchange | High | Moderate | Low |
| Treat After Deballasting (Port Facilities) | High | Low | High |
| Ballast With Treated Water | High | Low | High |
| Filtration And Cyclonic Separation | High | Moderate | Low |
| Filtration/UV Radiation | High | Low | High |
| Heat – Thermal Treatment | High | Low | Moderate |
| Microwaves | High | Low | High |
| Ultrasound | High | Low | Low |
| Electric Pulse | High | Low | Low |
| Biocides (Chlorine, Ozone) | Low | Moderate | High |
| Deoxygenation | High | Low | Moderate |
| Electro-Ionization Magnetic Separation | High | Low | Moderate |
| Photocatalysis/Ozone (UV/ AgTiO2 +O3) | Moderate | Low | High |
| Table 1: Evaluation of the current ballast wate r treatment technologies | |||
Therefore, antimicrobial heterogeneous catalysis using silver whose antimicrobial properties are already known [20-29], seems as an ideal solution for the disinfection of ballast water [30,31]. Silver (Ag) is among various other metals such as mercury and tellurium, which are not necessary for the proper functioning and the integrity of microbial cells [23,25]. Contrariwise, these metals are classified as highly toxic to cells even at extremely low concentrations since the defensive mechanisms which are used by every single cell are overcome by the activity of meal ions [32,25,27]. The remarkable microbicidal activity of silver reinforces its use on a variety of applications and products such as biomedical applications, air purification, cosmetics, clothing and water treatment [23,24,27]. Although the mechanisms of action of silver are not yet fully clarified, its antimicrobial activity mainly lies on the creation of silver ions (Ag+), the generation of excess reactive oxygen species (ROS) and the inhibition of several cell’s genes expression [25,27].
Silver ions resulting after the oxidation of Ag0, could interact with key enzymes and proteins, causing the inhibition of metabolic pathways, or induce the formation of pits in the extracellular membrane resulting cell lysis [20,24,25, 27]. Furthermore, silver ions promote the generation of excess reactive oxygen species (ROS) which apart from the penetration inside the cell where they can cause oxidative stress or several DNA damages and cell death in expansion, they can also lead to the observation of severe morphological changes in cells outer membrane [20,24 27].
Thus, recognizing that disinfection of water is one of the main selection criteria for every ballast water treatment method, this work attended the examination of the possible antimicrobial properties of silver supported catalysts using E. coli (Gram-negative) [33] and E. faecalis (Gram-positive) [34] as indicator microbes [35], according to the regulation D2 of IMO’s BWM Convention [5].
In the present work, five monometallic supported catalysts, 0.05 wt% Ag/γ-Al2O3 , 0.1 wt% Ag/γ-Al2O3 , 0.2 wt% Ag/γ-Al2O3 , 0.5 wt% Ag/γ- Al2O3 , and 1 wt% Ag/γ-Al2O3 , were prepared and examined for the antimicrobial treatment of ballast water. The aforementioned catalysts were prepared via a modified wet impregnation method. Initially, commercial γ-alumina spheres (Sasol, code 604130), were immersed in a solution containing specific amount of the compound AgNO3 (Panreac, code 131459), precursor of the Ag metal, dissolved in deionized water which was used as a solvent, in order to obtain the desired loading for each catalyst. Alumina spheres were left in the above solution for 24 h under mild stirring, followed by the evaporation of the solution within 6 h (pH=8). Consequently, the catalytic spheres were mildly dried at 120°C before their calcination in air at 400°C for 2 h.
The physicochemical properties of the catalysts and the effectiveness of the described alumina-coating procedure were thoroughly examined and reported previously, using a series of advanced analytical techniques [36,37]. The present catalysts were characterized in terms of actual metal loading using Atomic Absorption Spectroscopy (AAS) and internal surface area using the Brunauer-Emmett-Teller (B.E.T.) technique.
Catalytic experiments were conducted in a semi-batch mode using a three-phase continuous flow stirred tank reactor (Autoclave Engineers, USA, and PID Eng & Tech, Spain) equipped with a Mahoney-Robinson catalyst basket, which was designed in order to ensure the maximum contact area between the three phases and to reduce as much as possible, mass transport phenomena [37,38]. The gas phase oxidizing medium (20% O2 /80% He) was kept at a continuous flow while solid and liquid phases were stationary
For each experiment, a pre-weighed amount of catalyst was placed in the reactor’s basket where its in situ pre-treatment in terms of calcination in air at 400°C for 2 h, was performed. After temperature’s decrease to 25°C, the appropriate microbial solution was inserted in the reactor with a constant flow of 24 mL/min for 8 min, under continuous flow of He gas. The desired homogenized microbial solution for each experiment was prepared through the dissolution of the proper commercial lyophilized pellet using a sterile swab, in 0.5 mL deionized and sterilized water. Appropriate dilutions of the initial solution were performed in order to have a microbial load within the range of 30-300 cfu for every collected sample of the treated solution at predetermined intervals. At least five samples were taken for each catalytic experiment. Specifically, every treated sample was taken from the outlet of the reactor and it was transferred on a proper commercial selective medium which was able to develop only the bacteria contained in the treated solution. E. coli bacteria were distributed on the corresponding plate using the spread plate method with an inoculum of 0.1 mL for each sample, while E. faecalis were distributed on the corresponding plate using the membrane filtration method, where the filtration of 10 mL of treated microbial solution through specific filters was conducted for each sample.
The volume of the liquid phase (180 mL), the flow rate of gas feed stream (100 mL/min), the feed stream composition and the stirrer’s rotation speed (150 rpm) were kept constant in all catalytic experiments. In addition, most of the important experimental parameters such as the temperature, the pressure, the flow of the gaseous feed mixture and the volume of the solution in the reactor, were monitored through a fully computer-controlled panel.
The calculation of the bacterial inhibition caused by the catalyst in use was feasible through the counting of the developed colonies within 24 h and 48 h respectively. Furthermore, in order to ensure that bacterial inhibition was due to the bactericidal activity of the prepared monometallic catalysts, several blank experiments were conducted using sorely alumina spheres instead of catalyst. Additional experiments were performed with neither alumina spheres nor catalyst, for comparative reasons.
The actual Ag loadings in the Ag/γ-Al2 O3 catalysts were determined according to the results derived from Atomic Absorption Spectroscopy (AAS) (Table 2). Based on the results obtained by AAS, the Ag loading of each catalyst did not abstained significantly from the nominal values (Table 2). In addition, the specific surface area of the catalysts was found to vary from 194 m2 /g for the catalyst with the highest metal loading, to 199 m2 /g for the catalyst with the lowest metal loading, indicating that metal impregnation did not affect significantly the surface area of the support.
| Catalyst sample | Nominal Loading (wt%) | Actual Loading (wt%) | B.E.T. (m2/g) |
Pores Volume (mL/g) |
| 0.05 wt% Ag/γ-Al2O3 | 0.05 | 0.048 | 199.2 | 45.8 |
| 0.1 wt% Ag/γ-Al2O3 | 0.10 | 0.096 | 198.7 | 45.1 |
| 0.2 wt% Ag/γ-Al2O3 | 0.20 | 0.21 | 196.5 | 45.0 |
| 0.5 wt% Ag/γ-Al2O3 | 0.50 | 0.52 | 195.4 | 44.9 |
| 1 wt% Ag/γ-Al2O3 | 1.00 | 0.97 | 194.3 | 44.6 |
Table 2: Surface area and actual metal loading of the prepared catalysts
Aiming at a first and general evidence of the bactericidal activity of the newly-synthesized catalysts, 4 g of the catalyst with the highest metal loading (1 wt%) were initially used for the treatment of 180 mL of microbial solution which contained E. coli bacteria. The results revealed (after several experiments) that after 5 min of treatment, a total inhibition (100%) of the E. coli bacteria was observed, verifying at the same time that silver is a powerful antimicrobial agent.
Figure 1 presents the percentage E. coli bacterial inhibition with reaction time on stream, as a function of silver metal loading. As shown in Figure 1, the catalyst with the highest metal loading (1%) exerted total inhibition of E. coli in the very early stages of the solution’s treatment, in contrast with the lowest metal loading catalyst which presented total inhibition of E. coli at significantly higher reaction time. In addition, a total inhibition obtained for all catalysts after 20 min of treatment, disclosing the significant antimicrobial activity of all the catalysts prepared. It is also worth mentioning that reaction is favored at higher silver loadings. Moreover, for the better evaluation of the catalysts performance with reaction time (gradual inhibition), 1 g of the catalyst with the lowest silver loading (0.05 wt%) was used for the rest of the catalytic experiments.

Figure 1: E. coli bacterial inhibition profile as a function of Ag metal loading and reaction time. Reaction conditions: mcat =4.0 g; P=1.3 atm; T=25°C; Stirring Rate=150 rpm.
Figure 2 presents the percentage E. coli bacterial inhibition as a function of reaction time, using 1 g of the catalyst with the lowest silver loading for the treatment of 180 mL microbial solution. As Figure 2 reveals, nearly total inhibition of bacteria was observed within 2 h of treatment. Additionally, in order to evaluate the contribution of the catalyst support to microbial inhibition and to prove that the observed microbial inhibition is due to silver antimicrobial activity, 1 g of γ-alumina spheres was used instead of catalyst, to treat the same volume of microbial solution. Thus, as also illustrated in Figure 2, despite a slight antimicrobial activity presented by γ-Al2 O3 , the inhibition of bacteria is clearly lower than that obtained in the case of catalyst treatment. Moreover, the not insignificant antimicrobial activity exerted by the support may be due to the adsorption of bacteria to alumina’s surface which caused their removal from the solution. In particular, the percentage of inhibition after 120 min of treatment using sorely γ-alumina spheres was 28.5% as compared to 99.3% inhibition using equivalent catalyst mass. It should be also mentioned that almost no inhibition occurred after 120 min, when neither support nor catalyst used for the treatment of E. coli solution (blank experiment).
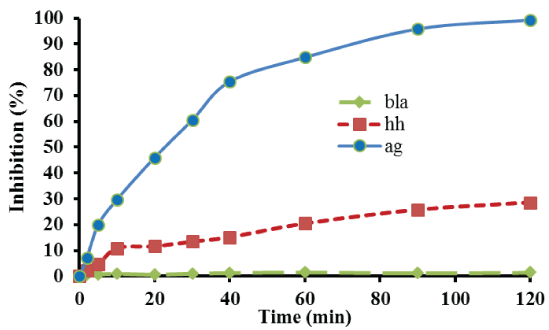
Figure 2: Detailed bacterial inhibition profile of E. coli. Reaction conditions: mcat=1.0 g; P = 1.3 atm; T=25°C; stirring=150 rpm.
Furthermore, Figure 3 presents the percentage E. faecalis bacterial inhibition as a function of reaction time, using also 1 g of the catalyst with the lowest silver loading for the treatment of 180 mL microbial solution. The results presented in Figure 3, reveal that E. faecalis inhibition after 60 min of treatment reached 76.2% compared to 11% microbial inhibition obtained when γ-aloumina spheres were used. As in the case of E. coli, almost no inhibition occurred after 60 min, when neither support nor catalyst used for the treatment of E. faecalis solution.
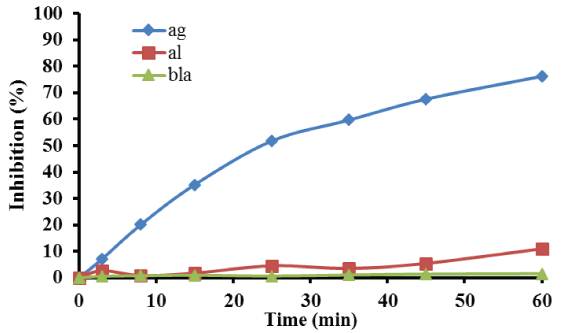
Figure 3: Detailed bacterial inhibition profile of E. faecalis. Reaction conditions: mcat = 1.0 g; P = 1.3 atm; T=25°C; stirring=150 rpm.
The results presented in Figures 1-3 clearly indicate that the prepared monometallic catalysts exert their antimicrobial activity within a short period of time, while it could easily be argued that ionic silver (Ag+) is responsible for the observed microbial inhibition. It is also noted that increased microbial inhibition obtained for gram-negative E. coli bacteria as compared to the inhibition obtained for gram-positive E. faecalis bacteria, may be due to the fact that gram-positive bacteria have a weaker negative charge than gram-negative bacteria, which is attributed to the several morphological differences identified in their structure [39].
The present work provides incontrovertible evidence that the prepared silver supported catalysts exert their antimicrobial activity both on gramnegative and gram-positive bacteria. It was also found that the reaction (bacterial inhibition) was favored at higher silver (Ag) loadings.
The results of the present work provide strong evidence, for the first time ever, that Ag retains its antimicrobial properties even when immobilized (deposited) on a solid support, a fact that may trigger a whole new field, that of antimicrobial heterogeneous catalysis.
Antimicrobial heterogeneous catalysis may be a promising method for the antimicrobial treatment of ballast water since it constitutes a relatively inexpensive and environmental friendly method which ensures the proper functioning and sustainability of the aquatic ecosystem where the treated water is discharged. Simultaneously, adverse impacts for humanity caused by the irrational massive rejection of pathogens through ballast water discharge could be eliminated. However, since this is a very new field, there is ground for much work to be done such as for example the examination of other microbes/bacteria, the behaviour of the catalysts under real conditions and the investigation of bimetallic Cu2+-Ag+ catalysts.
Download Provisional PDF Here
Article Type: Research Article
Citation: Theologides CP, Theofilou SP, Savva PG, Olympiou GG, Costa CN (2017) The New Concept of Antimicrobial Catalysis: Disinfection of Ships Ballast Water. J Environ Toxicol Stu 1(1): doi http://dx.doi.org/10.16966/2576-6430.101
Copyright: © 2017 Theologides CP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access