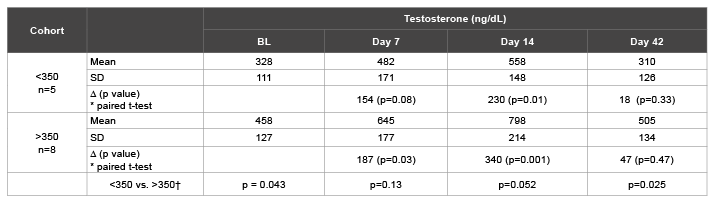
Table 1: Effects of treatment on serum testosterone in men with low normal and normal Levels †t-test

Ronald D Wiehle* Gregory K Fontenot Marcela Gomez Martinez Joseph Podolski
Repros Therapeutics, Inc., 2408 Timberloch Place, B-7, The Woodlands, Texas, USA*Corresponding author: Ronald D. Wiehle, Repros Therapeutics, 2408 Timberloch Place, B-7, The Woodlands, TX, 77380, USA, Tel/Fax: 281 719 3406; E-mail: rwiehle@reprosrx.com
Background: Clomiphene Citrate may be used off-label to treat men with secondary hypogonadism. More commonly used and approved are the exogenous testosterone products such as gels, patches, and injectables. One drawback may be the high levels of serum testosterone (TT) achieved with exogenous products. A single isomer of Clomiphene citrate, Enclomiphene citrate, is in development and may be useful and gain regulatory approval. An open question is whether men using Enclomiphene citrate will experience high levels of TT.
Aims: In our efforts to evaluate Enclomiphene citrate as an oral therapy for normalizing testosterone, we conducted a Phase I pilot clinical study (ZA-002: An Open Label, Fixed Dose, Single Center, Phase I Study to Evaluate the Changes in Total Testosterone with Oral Administration of Enclomiphene Citrate in Healthy Men with Low and Normal Testosterone) to assess the compound’s effects on TT.
Methods: Sixteen men, mean age 46, with low or normal testosterone levels were enrolled and 13 administered Enclomiphene Citrate drug for two weeks. The pharmacodynamics effects of Enclomiphene citrate on total testosterone (TT) were investigated. The Phase I study also assessed the safety and tolerability of Enclomiphene citrate in middle aged subjects.
Results: An increase in TT was observed in males with low baseline levels following 14 days of Enclomiphene citrate. The mean increase in TT ranged from147 ng/dL to 339 ng/dL and results in an increase into the normal range with few excursions above the upper limits. TT increases were greater in men with normal testosterone at baseline. After 14 days of Enclomiphene citrate, mean testosterone levels over a 24-hour sampling period were relatively similar with respect to Cavg. There was a trough in TT 12 hours after administration. TT levels returned to baseline within 28 days of the last dose of Enclomiphene citrate. No clinically meaningful effects on vital signs, laboratory safety tests or ECG results were noted.
Conclusions: Enclomiphene citrate is safe and effective in men with low and normal testosterone and rarely elevates TT above the normal range.
Secondary hypogonadism; Testosterone therapy
TT: Total Testosterone; FBG: Fasting Blood Glucose; GGT: Gamma Glutamyl Transaminase; LDH: Lactate Dehydrogenase; TC: Total Cholesterol; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; PSA: Prostate Specific Antigen; Hbg: Hemoglobin; Hct: Hematocrit; BUN: Blood Urine Nitrogen; RBC: Red Blood Cell; WBC: White Blood Cell; BPH: Benign Prostatic Hyperplasia, ED: Erectile Dysfunction; ECG: Electrocardiogram; SHBG: Sex Hormone Binding Globulin; BL: Baseline; Cavg: Average Concentration; PD: Pharmacodynamics.
Enclomiphene and zuclomiphene are structural isomers of Clomiphene citrate, a drug that has demonstrated both estrogenic and anti-estrogenic effects in women [1]. These differentials effects are thought to result from different activities of the enclomiphene and zuclomiphene [2] and a greatly extended half-life of zuclomiphene [3]. Clomiphene citrate has been used to induce ovulation [4] and also been used in men to raise testosterone [5,6] to reverse anabolic steroid abuse [7] and raise sperm counts in men with no-obstructive azoospermia [8].
Since enclomiphene has demonstrated anti-estrogenic activity and is postulated to normalize the function of the hypothalamic–pituitary– testicular axis observed in men with secondary hypogonadism, the compound may have utility as an alternative approach to raising or normalizing total testosterone (TT) levels in men with testosterone deficiencies. The increase in serum testosterone beyond the normal range is not without consequences [9,10]. In a recent report [11] where hypogonadal men were treated with injectable, topical and pellet formulations of testosterone, there was a difference in the level of TT and estradiol achieved and a clear difference in TT elicited by the injectable T product but with strong changes on erythrocytosis (changes in hemoglobin concentration and hematocrit). This may be contrasted with the use of Enclomiphene citrate to raise TT in the men with secondary hypogonadism [12-15].
At the screening visit, subjects underwent physical examination and 12-lead electrocardiogram (ECG). The ECG was performed to rule out cardiac dysrhythmias and define an otherwise healthy population. There have been reports that the use of exogenous T can lead to cardio-vascular events in older men [16]. Although men in the study were required to be 18-70 years of age, the age of the study population was 46.4 ± 12.5. Those men who were in the higher baseline TT group (>350 ng/dL) were 43.6 ± 9.6 and those in the lower TT group (<350 ng/dl) were 52.0 ± 16.9. There was no statistical difference between those two groups (p=0.23, t-test). The two groups did not differ in mean height (p=0.87, t-test) or mean weight (p=0.86, t-test). At screening, blood was drawn for laboratory analysis and a urine drug screen. The laboratory assays were done in the fasting state. Uncontrolled hypertension, a PSA >4.0, liver disease or a history of liver disease were exclusion criteria. The use of any testosterone, androgen, estrogen, anabolic steroids, DHEA, or a herbal product associated with hormones within two weeks of screening, any time during treatment, or the 28-day follow-up period were exclusionary. If subjects were sexually active, they were required to practice a highly effective method of contraception through the study, including the 28-day followup period. Vital signs (temperature, respiration rate, blood pressure [systolic and diastolic] and pulse) were measured and recorded and were required to be in the normal range. Thirty-one men were screened, 16 men were enrolled, and 13 men completed all parts of the study. The 24-hr pharmacodynamics study used 13 individuals. Fifteen men took medication at some point but two did not come to the last two visits.
After providing an IRB approved informed consent (Schulman Associates Institutional Review Board, 4290 Glendale-Milford Road, Cincinnati, OH 45242) and meeting all inclusion/exclusion criteria, subjects received study medication and were instructed to take two 12.5 mg capsules PO daily for 2 weeks. Treatment compliance was assessed based on daily diary cards (subjects recorded the dates and times of study drug dosing) and capsule count. Subjects returned to the clinic 7 days later (Day 7) so that total testosterone could be measured a second time. In addition, vital signs, adverse events and concomitant medications were assessed and recorded. Fourteen days after receiving study medication, subjects returned to the clinic. Blood samples were taken and the last study dose was administered in the clinic which was designated time 0. Subjects remained in the clinic for 24 hours and blood was drawn at the following time points: 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 and 24-hours post-dose. At least 0.5 ml of serum was collected for the TT samples. At the same visit (Day 14), the subject underwent complete physical examination, 12- lead ECG, concomitant medication review and adverse event review. All remaining study medication was collected. Subjects returned to the clinic 28 days later for a follow-up visit. The return visit was to insure recording of adverse events and was much longer than the treatment period because the effects of oral Enclomiphene citrate can persist in terms of LH, FSH, and TT after cessation of treatment [13]. Vital signs, adverse events and concomitant medications were assessed and recorded. In addition, blood was drawn for laboratory tests. Hormone analysis was performed by ABC Laboratories (7200 ABC Lane, Columbia, MO 65202) using a radioimmunoassay (RIA). Routine clinical chemistry laboratory evaluations were performed both before study qualification and after. Pre-established conditions that were verified but considered not exclusionary included the high lipid profiles, hypertension, BPH, ED, insomnia, and high FBG. Clinical serum chemistry analyses included liver functions, GGT, LDH, PSA, lipids (including TC, HDL, LDL, triglycerides), FBG, BUN, bilirubin, Hbg, Hct, RBC parameters, WBC counts, albumin, globulin, electrolytes and minerals including calcium. Urine analysis included color, specific gravity, WBCs, protein, glucose, bacteria, and hyaline casts.
This was a pilot study to establish whether significant changes from baseline could be found in men with low or low normal levels of TT who used Enclomiphene citrate. Protocol was approved by an extramural IRB as mentioned. There is and was ample evidence that men could see significant increases in TT beyond the normal range if they used products which contained T [9-11]. We had determined previously under IND 65,396 that Enclomiphene citrate used for 14 days was safe and able to raise TT in men with hypogonadism [15] in comparison to a topical T agent. Our previous study left open the question of effects in men who were not hypogonadal. No formal statistical determination of sample number was performed but it was anticipated that 15 subjects in three cohorts would provide sufficient data to determine changes from baseline. There were to be 3 arms of 5 individuals each but there were practical difficulties in finding subjects in the narrow 250-350 ng/dL range consistently and we decided to use only a low range (<350 ng/dL) and a high range (>350 ng/dL) group. The latter group of men was not hypogonadal and all men were otherwise normal. We limited the duration of treatment to 14 days, repeated a previously-used safe dose (25 mg per day) and employed a long follow-up period for safety considerations.
Mean values were determined. Differences between independent groups were assessed by the t-test or the non-parametric equivalent. Differences within groups over time or visits were assessed by the paired t-test. Differences over multiple t visits were assessed by ANOVA or the Kruskal-Wallis test.
We wanted to assess the effects of Enclomiphene citrate on men with low and normal TT. We wanted to determine, in particular, if the use of Enclomiphene citrate in such men results in excursions of testosterone out of the normal range. This is of interest because the lower cut-off value for normal testosterone has been subject to debate and may be influenced by assay used, the laboratory and the relative amounts of SHBG. Consequently, men in the normal range may be assigned to treatment erroneously. At the same time, men may raise their TT above the normal range and not be aware of the elevation. We have adopted the normal range here as being 350-1100 ng/dL.
Table 1 shows the effects of 14 days of administration of 25 mg of Enclomiphene citrate for men in the low (<350 ng/dL) and the normal (>350 ng/dL) groups. Subjects were chosen so as to produce a statistically significant difference in TT between the groups, a difference that was still present at baseline (BL) (p=0.043, t-test). The net increases in TT were 154 ng/dL at 7 days of treatment and 230 ng/dL after 14 days of treatment in the low group. The net increase in the normal group was 187 ng/dL after 7 days of treatment and 340 ng/dL after 14 days of treatment. These increases from baseline were statistically significant. Comparing two groups based on initial levels of TT, the effects of Enclomiphene citrate were stronger in men with a higher level of baseline TT. After 28 days of wash-out (Day 42), there was no difference in the TT levels for men compared to baseline.
The mean values for TT tended to increase with treatment and over time. At Day 7 the low group increased TT statistically compared to baseline (p = 0.08, t-test) and the normal group was also higher than baseline (p=0.03, t-test). At Day 14 both groups were higher than their baseline values. At Day 42 both groups returned to their baseline values and the normal group was higher that the low group (p=0.025, t-test). Two of the eight individuals in the normal cohort demonstrated values greater than 1100 ng/dL after 14 days, although such levels were not attained in the low cohort.

Table 1: Effects of treatment on serum testosterone in men with low normal and normal Levels †t-test
The mean group values for total testosterone measured every 2 hours over a 24-hour period following 14 days of daily Enclomiphene citrate administration are shown in Figure 1. At the initiation of the 24 hour PD experiment, men in the low cohort were determined to have a TT value of 558 ± 148 ng/dL and the normal cohort showed a value of 798 ± 214, a difference that suggested, but did not meet our definition of statistical significance (p=0.052, t-test). The mean Cavg values over the 24 hours were 519 ± 37 and 686 ± 55 ng/dL for the low and high groups, respectively. These values could not be shown to be statistically different (p=0.062, t-test). Mean TT levels in the low group and the high group were relatively steady with only a few individuals demonstrating skewed or kurtotic values over 24 hours. The individual Cavg values for the 5 subjects in low group varied from 393 ng/dL to 657 ng/dL whereas the individual Cavg for the 8 subjects in the high group varied from 492 ng/dL to 941 ng/ dL, both groups showing differences among individuals. Neither group was internally comparable over the PD investigation period by ANOVA or Kruskal-Wallis. TT differences between the two groups were observed at the 8, 10, 16, 18 and 20-hour time points, with higher values observed in the normal group. After 24 hours, TT levels returned to starting values, i.e., there was no difference between the initial (0 hour) and final (24- hour) value for TT for the low (p=0.89, paired t-Test) or the normal men (p=0.51, paired t-Test).
It was important to determine outliers during the 24 hour PD. The range of TT values is given. As shown in Figure 2, no individuals were determined to have a TT below 300 ng/dL and only 1 of 13 individuals was below 350 ng/dL. That single outlier was found in the low group and was <250 ng/dL at baseline. In the normal group, two individuals demonstrated values greater than 1100 ng/dL. One individual showed two excursions at 0 and 4 hours. The other individual had an excursion at 0 hours. Thus, individuals in the 24-hour time course study were within the normal range, 98.4% of the time.
As shown in Figure 2, mean TT for both groups ranged from 568.0 ng/ mL to 705.5 ng/mL over the 24-hour period with the highest mean occurring at Hour 0 and the lowest occurring at Hour 12. Testosterone values ranged from 382 ng/mL to 1269 ng/ml over the 24 hours (normal range 350-1100 ng/mL). The largest difference in individual testosterone values occurred at Hour 4 and the TT nadir was observed at 12 hours following administration for all subjects.
There were no deaths or serious adverse events during the study. Five adverse events (4 headaches and 1 instance of sleep disturbance) occurred in 2 subjects. All were mild in intensity and considered possibly/probably related to study medication. There were no meaningful changes in PSA over the study and all individuals were in the normal range. There were no clinically meaningful changes in vital signs, laboratory tests or ECG results.
This study of Enclomiphene citrate in subjects with low and normal initial TT showed increased levels of TT for both groups. As anticipated, mean change from baseline was highest at Day 14 and this trend was consistent (Table 1). We had previously determined that by Day 14 most clearly hypogonadal obese men with TT values <300 ng/dL realized an significant upward change in TT [14,16]. Figure 1 shows that the men in the normal group can expect to have higher values over 24 hours after 14 days of treatment. Despite these increases, mean testosterone levels in both cohorts remained essentially within normal limits during the Day 14 PD 24-hour monitoring period (Figure 2). After 14 days of Enclomiphene citrate, mean testosterone levels over a 24-hour sampling period were relatively similar. By Day 42, 28 days after discontinuing study medication, TT had returned to baseline levels in all cohorts.
Daily administration of Enclomiphene citrate to otherwise healthy men for 14 days appears to increase TT regardless of baseline testosterone levels without excursions beyond the normal range. Although, males with normal total testosterone levels at baseline responded to daily doses of Enclomiphene citrate with an increase in TT greater than that observed in the low group, TT generally remained in normal or acceptable ranges. The daily rhythm of TT appears to be preserved in terms of a morning peak, a mid-day trough and a night time rise [17,18]. We have reported a mid-day trough in TT in hypogonadal men treated with Enclomiphene citrate [13].
We recognize limitations of this study. It was a pilot study following a Phase I study in secondary hypogonadal men that showed that men with low TT could expect elevations [15]. Although the number of subjects was limited, we were particularly interested in men with normal levels of TT, a group for which there was no previous experience. The dividing point between the low and high TT groups was set at 350 ng/dL to ensure capturing men for the high group who would have less chance of being latently hypogonadal. We did not evaluate men with low T for primary or secondary hypogonadism although men with primary hypogonadism would not be expected to respond to Enclomiphene citrate which works centrally by raising LH and FSH [12-15]. Indeed all the men in the <250 ng/dL group responded to drug. We used no placebo since comparisons were among men on the basis of their TT levels. Comparisons were made against baseline. We used a single dose to keep the protocol straight forward in our small population. Our single site was a limitation since we could not draw from a large patient base. Although we looked at a limited number of men, the 24-hour PD after 14 days of treatment represents a rigorous way to assess TT. We generated both a 24-hour profile and a Cavg. The 24 hour profile is valuable in the sense that TT can demonstrate a clear pattern over that time period, especially in younger men [13]. The Cavg provides an ideal way to estimate the TT in men who have variable hourly TT.
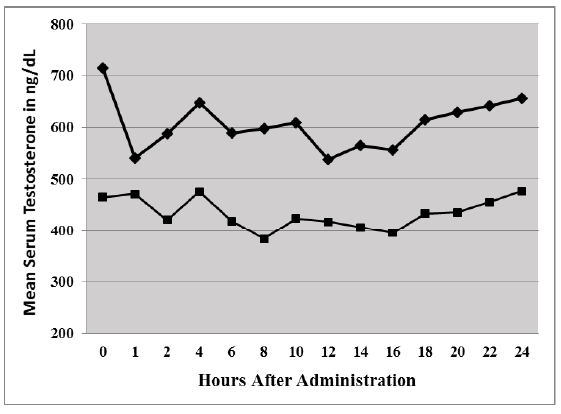
Figure 1: Twenty-four hour PD profile. Pharmacodynamics of Enclomiphene citrate. Men with low serum testosterone (filled squares) or normal serum testosterone (filled diamonds) at baseline are compared. After 14 days of treatment with 25 mg of Enclomiphene citrate, subjects were given a single oral dose and blood was drawn every two hour over the next 24 hours. Levels of TT were determined by RIA.
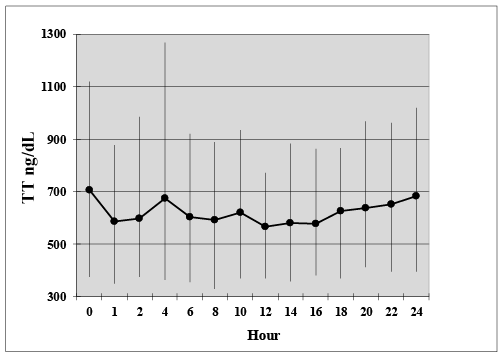
Figure 2: Range of Values over the 24 hour PD Variations in the values of serum Testosterone (TT) over the 24 hour PD. Following 14 days of Enclomiphene citrate treatment, subjects were assessed for TT every 2 hours. The points are the mean TT at each time point. The vertical bars demonstrate the range of each time point, i.e., highest and lowest TT values found.
Data from previous studies conducted in men with secondary hypogonadism suggest that daily administration of Enclomiphene citrate is safe and effective for men in the low range. The present data suggest for the first time that daily Enclomiphene citrate is likely to be safe and effective for men with low or normal TT, but additional study of safety factors would be warranted. Such additional studies may support the use of Enclomiphene citrate as an alternative to currently available testosterone replacement therapies associated with supraphysiologic levels of testosterone.
Fred Lowery, DVM, as the Responsible Medical Officer is recognized. The Principle Investigator, Mark Kalish, M. D. at Texas Urology Specialists, Memorial Urology Associates Memorial City, 915 Gessner, Suite 720, Houston, TX 77024 is recognized.
Download Provisional PDF Here
Article Type: Research Article
Citation: Wiehle RD, Fontenot GK, Martinez MG, Podolski J (2015) Oral Administration of Enclomiphene Citrate Results in Physiological Total Testosterone Levels in Men with Low or Normal Testosterone: A Pilot Study. Int J Endocrinol Metab Disord 1(3): doi http:// dx.doi.org/10.16966/2380-548X.114
Copyright: © 2015 Wiehle RD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access