Figure 1: Multivariate representation of subjective physical and mental condition of OSAHS before and after CPAP


Hamid R Hosseini1 Adil O Mageet2,3*
1Resident, Department of Orthodontics, HBM College of Dental Medicine, Mohammed Bin Rashid University, Dubai, UAE*Corresponding author: Adil O Mageet, Department of Orthodontics, Hamdan Bin Mohammed College of Dental Medicine, Mohammed Bin Rashid University, Dubai Healthcare City, Dubai, UAE, E-mail: amageet2000@yahoo.co.uk
Obstructive (Peripheral) Sleep Apnoea Hypopnoea Syndrome is an increasing breathing problem, caused by repetition of upper airway occlusion during sleep due to anatomical or pathophysiological factors. There are wide ranges of treatment available for this condition, both surgical and non-surgical procedures. This review article intends to facilitate and improve understanding of the non-surgical treatment of this disorder. The article intends to group all non surgical modalities together and put them into a format accessible to undergraduate dental and medical students, residents, general dentists, general physicals and specialized dentists.
Obstructive sleep apnoea; Intra-oral appliances; Snoring
The management of Obstructive Sleep Apnoea Hypopnoea Syndrome (OSAHS) depends on the severity of symptoms, the extent of clinical complications and the aetiology of the upper airway obstruction; therefore treatment is divided into non-surgical and surgical procedures. In this review article detailed information about non-surgical treatment options is elaborated.
Patho-physiology involves a number of factors that potentially aggravating OSAHS. Co-existing chronic obstructive airway disease, asthma, and hypothyroidism are known medical conditions exacerbating OSAHS. Adequate therapeutic control of these factors is therefore important in managing the condition. The elimination of aggravating factors, e.g. intake of alcohol and other CNS depressants including sleeping tablets, will make the airway more prone to collapse during sleep; therefore reducing alcohol intake, particularly during the evening, is sensible, if not always palatable advice [1].
Those patients addicted to regular smoking should be encouraged to reduce or cut the habit, together with the avoidance of alcohol, sedatives or sleeping tablets. While advice should be given to Non-sleepy snorers not to sleep on their backs [2].
These measures may be adequate for simple snorers or sufferers with very mild OSAHS and few symptoms; however most patients with OSAHS require additional treatment.
A Cochrane review of lifestyle modifications for OSAHS found a lack of evidence from randomised clinical trial (RCT) to support the use of these and advised against delaying implementing therapies of proven effectiveness, such as CPAP while such unproven methods were tried [3].
Recent guidelines (American Academy of Dental Sleep Medicine, 2015) [4] recommend:
Also, the American Academy of Sleep Medicine (AASM) [5]makes the following recommendations regarding OSA:
Furthermore, obesity exacerbates OSAHS; fatty deposits both subcutaneously and in the pharyngeal wall lead to further narrowing of the oropharynx, encouraging its occlusion once the subject is supine. Because many OSAHS sufferers are obese, weight reduction is frequently suggested. This change in lifestyle is far from easy to accomplish and many individuals are insufficiently motivated. For those who are committed, dietary advice should be provided. Once the weight has been lost, regular exercise is easier and this is in the subject’s overall medical interests. However, although the subject may feel fitter and less lethargic when his weight is within normal limits, his apnoea may not show a parallel improvement [1].
OSAHS is associated with morbidly obese patients and showed that treatment with a dietary regimen significantly improved or occurred 55% of the patients with a mean reduction in BMI of 27% [8].
The SIGN report (2003) on OSAHS stated that: Weight loss should be encouraged in all patients with obesity contributing to their OSAHS, attempt at weight loss should not delay the initiation of further treatment and Weight loss should also be encouraged as an adjunct to CPAP or intra-oral devices as it may allow discontinuation of therapy [2].
Lifestyle changes can have efficient effects in modifying the symptoms of pediatric OSAS. The prevalence of childhood obesity is increasing nowadays [9,10]. A thorough weight reduction program is an important step for pediatric patients that are obese or overweight. Weight loss has been suggested on the basis that it should decompress the upper airway and promote its patency, especially if weight gain is coincident with the aggravation of the symptoms [3]. OSAS may aggravate gastro- esophageal reflux or vice versa. Children with severe sleep apnea should avoid eating large quantities just before bedtime, particularly in cases where children are being treated with CPAP that can lead to air swallowing and gastric distention. Nevertheless, there is a scarcity of data concerning the effects of weight loss on OSAS in children and adolescents. Lastly, along with many other health-related benefits, achieving weight loss and increasing exercise and dietary management can have beneficial effects for OSAS and should be advocated along with other interventions for OSAS in obese pediatric patients [11-13]. Other lifestyle changes are intended to improve sleep hygiene, which is extremely important for individuals. Some of these changes involve measures to improve the sleep environment (child’s bedroom should be cool, quiet and comfortable), improving the sleep - wake patterns, increasing physical activity during the day, preparation for sleep by mentally winding down and avoiding daytime naps and caffeinated drinks in the evening and other stimulants drinks. Avoidance of using alcohol and drugs that suppress respiratory reflexes is also important in adolescents because of relax of pharyngeal muscles allowing the pharyngeal walls to collapse more easily. Also smoking results in swelling and irritation of the pharyngeal space, increasing the possibility of snoring and OSAS. There have been a few clinical studies in an adult population of sleep hygiene, but sleep deprivation has been revealed to increase the collapsibility of the upper airway. However, it remains uncertain how efficient they are in decreasing symptoms [3,14].
A Training of pillow attached to the sleeper’s back by a belt around the waist or a tennis ball sewn into the pajama top at the mid-back level are probably the two most commonly employed devices. Posture alarm could be tried [15]. Cartwright and his colleagues trained 10 known OSAHS patients to avoid the back sleep position by wearing a gravity-activated position monitor/alarm on the chest. A device emitting an auditory signal if the patient remained supine for more than 15 seconds. They found the number of apnoeic events was significantly reduced, together with the frequency of episodes of significant O2 desaturation [16].
Many retrospective studies assessed the effect of body position during sleep on OSAS in the paediatric population. In one study it was revealed that young children had an increased AHI in the supine position; yet in another study, they did not found a positional change in AHI [13,17]. No study evaluated the effect of changing body positions or the feasibility of maintaining a child in a certain position overnight. Hence, at the present, there are no recommendations that can be made with respect to positional therapy for OSAS in children [18].
Greater stimulation of inflammatory processes and oxidative stress have been suggested to explain the morbid consequences of sleep disordered breathing that may be further modified by lifestyle, genetic and environmental factors. Consequently, it is now being investigated to use nonsurgical approaches for children with OSAS in order to target those inflammatory processes. Pharmacological therapy is usually used for mild forms of this syndrome (AHI<5 events/hour), in children with accompanying allergic diseases and for residual obstructive sleep apnea syndrome [19]. There is still debate about the likelihood of using topical nasal steroids and leukotriene antagonists. An older study did not demonstrate a therapeutic effect of systemic corticosteroid use in this condition [20]. Only a few studies evaluated the use of local nasal corticosteroids, leukotriene antagonists or combination of both in children with OSAS retrospectively. This treatment can improve symptoms (reduce mucosal edema and volume of adenoids and tonsils) of mild forms of OSAS and children with allergy, but with small clinical effects. On the basis of these studies, intranasal steroids may be considered for treatment of mild OSAS (AHI<5 events/hour), but should not be used as the primary treatment of moderate or severe OSAS. The long-term effects of intranasal steroids are no well-known, follow-up evaluation is required to monitor for the adverse effects and to ensure that the OSAS does not occur again. There is absent of studies that particularly evaluated children who had atopy or chronic rhinitis, while one study mentioned that similar improvements were seen in children who had a history of allergic symptoms compared with those without [21,22]. There is a need for further study to determine whether children who have atopy are more likely to respond to this therapy [23]. Good nose hygiene and the lavage of the nasal cavity with the hypertonic solution are recommended as an adjunct local treatment particularly in mild forms of OSAS. Systematic and local inflammation can also contribute to the increased resistance at the adenotonsillar level in children. Local and systemic activations of leukotrienes and corticosteroid receptors play a significant role in the pathophysiology of this syndrome in children, there are not enough data to conclude whether the inflammatory mechanisms are a component of the cause of OSAS or rather a consequence of the recurrent upper airway collapse and mechanical trauma [19,24,25].
There is only a small body of accumulated evidence supporting pharmaceutical therapy as an effective therapeutic choice. The most significant systematic reviews of pharmacotherapy arrived at the conclusion that there was a lack of any medication demonstrating a consistent response [26,27]. Pharmaceutical agents may sometimes relieve symptoms characteristic of OSAHS. On the whole, these therapies aim to increase glossopharyngeal neurologic activity or reduce REM sleep.
A limited amount of evidence exists supporting that adding alerting drugs, for example, modafinil can positively affect sleepiness to a very limited degree in some patients who, in spite of good CPAP compliance persist in sleepiness. Nevertheless, these substances may enable a reduction in CPAP use, but longer-term studies of their beneficial effect and inherent risks are needed [28]. No extant evidence suggests any realistic prospect of their being used as an alternative to CPAP nor that constitute an alternative to diligent attempts to improve CPAP comfort and effectiveness.
Medications most frequently prescribed for the treatment of the condition include protriptyline and theophylline. Unfortunately, these drugs are unsuitable for many subjects and of unproven efficacy. The search for a more appropriate alternative is on-going.
The overwhelming opinion suggests that pharmacological therapy is unsuitable as a first line of therapy for OSAHS [2].
Protriptyline (a non-tricyclic antidepressant), the most commonly used and the most studied. It may be associated with a mild reduction in apnoeic episodes [29]. Its initial introduction was as a treatment for OSAHS on the basis of its ability to lower the frequency of apnoeic events and oxygen desaturations during non-REM sleep, while simultaneously suppressing REM activity (that stage during which the apnoeas tend to last the longest). Subsequent investigations uncovered an additional benefit: enhancing the tone of the upper airway musculature [30]. Unfortunately, severe anticholinergic side effects from taking the drug occurred in about half the subjects of the observed [31]. The same researchers investigated the effectiveness of theophylline in OSAHS patients, and recorded a reduction in the total number of apnoea and hypopnoea events, as well as in AHI, unfortunately, there was a simultaneous and significant deterioration in sleep quality [31] and, there seem no trustworthy means of predicting the patients who are likely to benefit from theophylline, although those with mildly afflicted are thought likely to benefit most.
Nocturnal hypoxaemia in sufferers of OSAHS may contribute to the development of cardiopulmonary morbidity. Thus, prevention of hypoxaemia by Oxygen administration is a worthwhile therapeutic goal in these patients. Up to the present, the evidence indicates the provision of supplemental oxygen during sleep is unlikely to be adequately effective in reducing the frequency of apnoea and increasing daytime alertness as a sole therapy for the majority of patients [32].
Preliminary studies indicate that electrical stimulation of the submental muscle (genioglossus), which acts as upper airway dilator, will enhance luminal patency and reduce the number of obstructive apnoeas [33].
Patients with Rapid eye movement (REM) related OSA usually has an overall low AHI with a disproportionately lower percentage of REM sleep as a function of total sleep duration. But, it is still unknown if obstructive respiratory events that are different than REM sleep with a differential neurocognitive and cardiometabolic impact in comparison to events that happens in NREM sleep. It is now clear that REM sleep is related with greater sympathetic activity and cardiovascular instability in human subjects that are healthy and patients with OSA in comparison to NREM sleep [34,35]. The hemodynamic and sympathetic changes during REM sleep trigger a surge in the heart rate and the blood pressure. Acute hemodynamic changes might be involved in causing ischemic incidents in patients with cardiovascular disease [35,36]. Obstructive apneas and hypopneas during REM sleep can lead to hypoxemia at a greater degree [29,37] and greater levels of sympathetic activity in comparison to events in NREM sleep [34] Numerous studies have established an association between OSA and adverse neurocognitive and cardiometabolic results; there is a major gap of knowledge on the risks involved with REM-related OSA which are untreated.
Continuous positive airway pressure (CPAP) has been termed the ‘gold standard’ for treating OSAHS and was developed by Colin Sullivan, in Australia in 1981. It provides dramatic relief of symptoms and ensures airway patency during sleep [38] by elevating the pressure in the oropharynx. A continuous stream of air under low pressure is filtered and delivered to the pharynx via a nasal mask. This constant flow is sufficient to prevent the airway from collapsing regardless of the position of the subject, but not enough to prevent expiration. The mask must fit firmly round the nose and to secure it, a head cap and retaining straps must be worn. CPAP should be in place for 4 to 6 hours per night, seven nights a week [39,40].
A distinctive feature of CPAP therapy is the immediate and dramatic response. In most cases the patient begins to exhibit long periods of uninterrupted sleep immediately after initiation of treatment, show a marked rebound in stage 3 to 4 NREM sleep occurs, and both the frequency and duration of this stage sleep increase dramatically. This rebound continues over a number of nights, until, by the seventh to the tenth night, normal levels of distribution and reusability are generally achieved. The daytime function of subjects is often transformed; the loss of daytime somnolence is the most noticeable sign of improvement but all other symptoms of OSAHS may also be reversed.
Whilst very effective in most cases, it is appreciated that CPAP is often a difficult device to tolerate. The pump delivering the air stream is noisy, making a constant ‘humming’ reminiscent of an air-conditioning unit. The anti-social nature of the mask and the headgear coupled with cumbersome and sometimes impractical nature of the device are thought to be of significant importance with regard to its acceptance and compliance.
It has been estimated that long term compliance of user’s amounts to between 60% and 70% [1] While nCPAP provides air stream pressure sufficient to exert a splinting effect upon the upper airway, the air pressure is insufficient to prevent periodic expiration. This addresses the complaint voiced by a number of OSAHS users about finding expiration difficult with conventional CPAP. In situations where persistent difficulty with expiration is reported, alternative approaches include the use of Bilevel CPAP (Bi-CPAP), which utilizes a second tube allowing a separate channel for expiration, and a device incorporating a ramp type feature ensuring that pressure does not increase until the patient is already asleep. Interestingly, investigations have revealed no increase in compliance with these alternatives [40].
A major concern with nCPAP as a therapeutically effective method of treatment of OSAHS concerns long-term compliance of the subject. The use of the device in the longer-term requires a considerable commitment by suffers, and the patients most severely affected by symptoms linked with OSAHS are more likely to use it consistently. The overall long-term compliance has been estimated to be between 50 and 70% [41-44], with this found to fall to approximately 30% for mild cases [45]. Interestingly, when studies have been performed in which the actual use of CPAP is monitored ‘covertly’, the actual figure has been found to be significantly lower than the self reported one [46].
The reported benefits of nasal CPAP are: Improved quality of life; improved daytime cognitive function; reversal of daytime symptomsin particular, sleepiness; elimination of snoring and other night-time symptoms; and long term improvement in cardiovascular function, with reduction in blood pressure.
There are significant side effects to CPAP use (e.g. marked epistaxis, para-nasal sinusitis) but these are infrequent, however minor side effects (irritation connected to the mask, nasal bridge sores [47], nasal congestion, dryness or excessive rhinorrhoea, upper respiratory tract infections, abdominal bloating, discomfort, noise, chest pain, and feelings of claustrophobia) are much commoner. CPAP uptake of up to 95% [48] can be achieved by intensive efforts with the device being used nightly for three to five hours [49,50]. Mouth leaks allowing sizeable amounts of cool air through the nose are blamed for the majority of nasal symptoms. It is possible to reduce such leaks by the use of chinstraps or full-face mask [2]. Some patients respond to the use of nasal corticosteroids [2] while both comfort and compliance can be improved by using a heated humidifier [51].
The Scottish Intercollegiate Guidelines Network report (2003) for OSAHS, endorsed by the British Thoracic Society, has recommended that CPAP should be the therapy of choice for patients with moderate or severe OSAHS where the symptoms are severe enough to require intervention, Bi-level ventilation is recommended as reserved for OSAHS patients with ventilatory failure and persistent low CPAP use (less than two hours per night) over a period of 6 months; after patient comfort in such cases, the therapy plan should be reviewed [2].
The SIGN (2003) report for OSAHS also recommended against CPAP therapy being discontinued without: The attention of a trained CPAP nurse/technician, a titration study/use of auto titrating CPAP to troubleshoot problems and the use of heated humidification.
Over two thirds of patients who took CPAP, home continued with CPAP at 5 years of age, with an average nightly use of 5.7 hours [48].
Long-term predictors of CPAP use are snoring history, apnea/hypopnea index (AHI), and Epworth score, 86% of patients with Epworth >10 and an AHI ≥ 30 still using CPAP at 3 years of age which brings the conclusion that long-term CPAP use is related to disease severity and subjective sleepiness and can be predicted within 3 months of age (Figure 1).

Figure 1: Multivariate representation of subjective physical and mental condition of OSAHS before and after CPAP
Since Pierre Robin first described his monobloc appliance in 1902 for the treatment of glossoptosis (tongue falling back and occluding the airway) in infants, several appliances have been devised for treating upper airway obstruction. These are termed generically as ‘oral appliances’ when they are designed to be inserted into the mouth to modify the position of the mandible, the tongue and other structures in the upper airway in order to relieve snoring or sleep apnoea.
There are five basic categories of dental appliances used in the treatment of OSAHS: the mandibular repositioning appliances, the soft palate lifters, the tongue repositioners, the equalizers and the magnetic appliances (Table 1).
Like CPAP, Mandibular Advancement Appliances (MAA) are a noninvasive and therefore reversible form of treatment, and worn only during sleep. The rationale for the use of (MAA) is that they are able to increase the dimension of the pharyngeal airway by drawing the tongue and soft palate forwards, and in this way maintain its patency during sleep.
In 2000 Liu selected twenty-two patients with confirmed diagnoses of obstructive sleep apnoea obtained by initial nocturnal polysomnography. A mandibular repositioning device was fitted to all subjects with the aim of holding the mandible in a more antero-inferior position. After six months, an outcome polysomnographic study was carried out on each patient with the appliance in place. Lateral cephalometric radiographs in the upright position were made in addition both before and after 6 months of treatment. In 21 of the 22 patients, the apnoea hypopnoea index decreased when the appliance was in place. For the total 22 patients the mean respiratory disturbance index fell significantly from 40.3 to 11.7 events per hour (P <0.01). Treatment success was deemed for 59.1% of subjects whose follow-up respiratory disturbance index <10 events per hour. There was an additional significant improvement in the mean minimum blood oxygen saturation level during sleep from 73.4% to 81.3% (P <0.01) [52].
Mandibular advancement devices (MADs) are the main alternative to non-continuous positive airway pressure (non-CPAP) for patients with obstructive sleep apnoea (OSA). The aim of using these devices is to increase the size of the upper airway and decrease the risk of sleep apnoeas and snoring in patients suffering from OSA. The upper airway is enlarged mainly in its lateral dimension [53,54]. The pharyngeal fat pads reposition laterally from the airway and the tongue base muscles move anteriorly [53]. This will cause a reduction in pharyngeal collapsibility [55]. There are suggestions that MADs cause a change in muscle activity during sleep, with the relaxation of the genioglossus muscle throughout incremental mandibular advancement [56] and the activation of the masseter and submental muscles [57]. MADs have been suggested for patients with mild to moderate OSA who prefers an oral appliance to CPAP, provided that the MAD has an adequate effect [7,58]. MADs can also be used in patients with the more severe disease who do not respond to or who fail treatment with CPAP. Some studies have recommended that the efficacy of MADs in changing the health risks associated with OSA is somehow similar to that of CPAP [59]. The treatment result of MADs has to be proved in a renewed sleep apnea recording with the device in all patients with OSA [57], since patients may have a positive treatment response. One general limitation of MAD treatment is its reliance on oral health and the fact that it takes some time to become adapted to the device. Side effects from the treatment, such as pain from the teeth and jaws, are generally mild and transient [58,59]. In the longer span, bite changes develop more common, but these are mostly minor and do not disturb patients who are pleased with the treatment outcome in terms of snoring and daytime symptoms [59].
Johal et al. in 2007 [60] recommended that DISE with associated mandibular advancement maneuver to mimic the treatment effect can have prognostic values in defining the possibility of successful mandibular advancement splint therapy in subjects with sleep-related breathing disorders. Other authors in the literature have supported the idea of DISE with the addition of a simulation bite [61]. Vroegop et al. in 2013 [62]revealed that patients in whom upper airway patency improved considerably with the presence of the simulation bite in maximal comfortable protrusion during DISE are more likely to be treated successfully with MAD treatment.
There is a simple explanation for the effectiveness of these appliances: the tongue is prevented from collapsing against the posterior pharyngeal wall nocturnally by the MMA. The mechanical achievement of this because the origin and insertion of genioglossus occur at the hyoid bone and the mandibular symphyseal region, respectively. Thus, the mandible is advanced; the tongue is restrained nocturnally in a more anterior position so as to increase the airway space.
In human beings, the voluntary passive opening of the mandible significantly increases genioglossus EMG by activating receptors in the temporomandibular joint [63]. This contraction of the genioglossus effectively opens the airway thus alleviating any tendency to airway obstruction. There have also been suggestions that the added vertical dimension produced by these appliances also increases the tongue tonicity so as to decrease the risk of airway occlusion [64].
The mandibular repositioning appliance has many different variations. It is typically constructed of clear acrylic resin, together with retentive Adams’ clasps.
Guidelines exist for the amount of forward movement thus produced. This is proposed to be optimal at between 50% and 75% of the patient’s normal maximum protrusive distance. This can be maintained using of a one-piece or fixed appliance holding the maxilla and mandible together, clasps of acrylic or other thermoplastic polymer construction being utilized to achieve this. This protrusion inevitably produces some concomitant opening, so care must be taken to ensure there is no rotation of the mandible downwards and backward produced by appliances [63]. An important feature of this appliance is that anterior air holes are necessary to facilitate oral respiration, especially in cases of restricted nasal airflow. Common used designs include the cribbed activator [65] vacuum-formed devices, the Nocturnal Airway Patency Appliance [66] and the Sleep Nocturnal Obstructive Apnoea Reducer [67].
In figure 2 Soll and George described a modified activator (Nocturnal airway patency appliance, NAPA) that advanced the mandible 6 mm anteriorly and 9 mm inferiorly for one patient and significantly reduced the AHI [66]. The appliance has eight Adams clasps with overlapping acrylic on the facial and lingual surfaces of the teeth. It is designed to protrude the mandible about three quarters of the distance between centric occlusion and full protrusion. The mandible is opened vertically just enough to permit an airway between the incisors. The NAPA rigidly stabilizes the mandible in both the horizontal and vertical dimensions. The effects of the NAPA in reducing the AHI have been documented in subsequent studies [68,69].
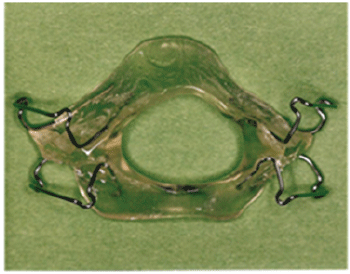
Figure 2: Mandibular Repositioning Appliance
The Sleep and nocturnal obstructive apnoea reducer (SNOAR) is an open airway acrylic mandibular advancement appliance that advances the mandible 6 to 9 mm and opens it vertically 17 mm or more. The mean AHI was reduced from 45.5 to 9.7 and snoring was absent after the SNOAR appliance was inserted [67].
This prefabricated Snore guard (Dental orthosis) appliance positions the mandible 3 mm behind the maximum protrusion with 7 mm opening. It covers only the anterior teeth and is lined with a soft polyvinyl for patient comfort. It is easy to fit and adjust directly on the patient and appears to be well tolerated.
In two initial studies [70,71] snoring was decreased significantly or completely eliminated. Later reports found a significant decrease in AHI, particularly among mild apnoea sufferers [72,73].
In cases of active bruxism and/or where the patient feels restricted by the rigid fixation of the jaw with the one-piece design, an alternative, more ideal, design involves constructing separate maxillary and mandibular appliances. The connection between the upper and lower appliances is achieved using inter-arch elastics and buccal tube and rod attachments (e.g. Herbst appliance, Jasper Jumper), or a single hook and latch in the anterior region (e.g. Thorton Adjustable Positioner). The aim of this design is the restriction of all retrusive movements while permitting the patient movement of the mandible both forward and side to side, in addition to being able to open the mouth if necessary.
Two-piece appliances have the additional advantage of enabling the systematic pinpointing of the exact mandibular position providing maximum benefit to individual patients. This is best done by initial setting at 50% of maximum protrusion at the first appointment and subsequently progressively advancing the mandible unit until the elimination of all signs and symptoms. On the other hand, these appliances, because of their construction, may be difficult for the subject to manage.
The Herbst Mandibular Advancement Splint can be justified as a choice for selected subjects experiencing sleep-related breathing disorders [74].
Only one preliminary evaluation of the Jasper jumper and twin block appliances for the treatment of OSAHS has been completed [75], in which 11 subjects with varying degrees of OSAHS were evaluated. Seven of the 11 subjects tested before and after appliance, insertion had reduced AHI values, but there were no significant differences. Even after vertical elastics were added, only half of the subjects tested showed a reduced AHI. This appliance may be more easily tolerated than the more rigid mandibular advancement appliances, but additional studies with varying mandibular vertical and anteroposterior positions are required to verify its usefulness.
Tongue repositioners are two types either tongue retainers or tongue trainers. Tongue retainers subdivided into tongue retaining device (TRD) and tongue locking device (TLD). The TRD aims at repositioning the tongue in a more anterior position during sleep so as to reduce the risk of obstruction at this level [76]. The device uses negative pressure in a soft plastic bulb to locate the tongue; a flange, fitting between the lips and teeth, which act to hold the device and tongue anteriorly in the oral cavity. It is important to note that this appliance, kin addition, modifies mandibular pressure in terms of forward rotation. The TRD is normally produced from dental impressions, however a prefabricated version is now available capable of being molded to the patient’s teeth in the clinic; which is also suitable for use with edentulous patients. A modified TRD with lateral airway tubes is also available to suit patients with obstructed nasal passages. The disadvantage of the TRD is that the tongue is not always held forward because surface adhesion of the tongue in the bubble is lost over time, and the patient must then awaken and relocate the tongue into the bubble. An aesthetic drawback is that the tongue has to protrude slightly between the teeth.
The TRD is the only appliance that has been studied in various body positions and in conjunction with other forms of therapy [77]. The TRD appears useful, either alone or in conjunction with other treatments, for improving patients with a wide range of apnoea severity provided that the apnoea is more severe in the supine position and the patient’s weight is not greater than 50 percent of the ideal [64].
Compared to the most commonly performed CPAP, the TRD is the more easily tolerated and has fewer long-term compliance problems.
This Tongue locking device (TLD) is a simple patented preformed elastic appliance available in small, medium and large sizes that provide a cavity for the tongue and holds it forward with a self-created vacuum during sleep. Lateral breathing holes assist airflow if nasal obstruction occurs. The TLD is simple and inexpensive to fit directly on patients.
Prinsell and his colleagues studied 10 OSAHS subjects using the device and found that five individuals had a reduction in AHI and five subjects became worse [77].
Tongue posture trainers come in two types the Tepper oral proprioceptive simulator (TOPS) and the tongue positioner and exerciser (TPE).
This (TOPS) appliance is fitted to the maxillary arch with a posterior tongue extension held inferiorly by an elastic band. An anterior padded bar, lingual to the incisors, is included to guide correct tongue placement. According to Dr.Tepper: its primary use is for those patients who snore, have apnoea, have problems of abnormal tongue posture and/or function and for those who have loss of muscle tonus of the soft palate and pharynx. All these abnormalities are corrected by proprioceptive means through the receptors being stimulated by the hinged portion of the appliance resting on the dorsum of the tongue. By increasing the resistive force of the elastics, the dorsal muscles of the tongue are also strengthened. Thus by correct repositioning of the entire tongue to the hard and soft palate; the idea is to increase airway space. Published data on its effectiveness for the treatment of OSAHS are not yet available.
The (TPE) is a custom-made appliance that has been used to treat snoring. Patients are trained to position the tongue above the ramp; according to the inventor, this ‘retrains the tongue and lip musculature to be in the proper rest and saliva swallowing position’. Published results before and after appliance insertion are not yet available.
Paskow and his co-worker in 1991 invented the adjustable soft palate lifter (ASPL) [78]. The appliance is designed to lift the soft palate gently and prevent it from vibrating in the air passage during sleep. The ASPL consists of a maxillary removable appliance with two Adams clasps on the molars and an acrylic button extending distally to the midpoint of the soft palate. Patients who gag are ‘desensitised’ with palatal exercises involving contact with the end of a spoon or toothbrush five or six times a day. Paskow claimed 60 percent success rate for snoring but felt the appliance was not indicated for the treatment of OSAHS. However, other researchers found that soft palate lifters were insufficient for treating snoring [80].
The equalizer appliance was introduced by Haze in 1987; it is constructed of a vinyl material and repositions the mandible in a position of “neuromuscular balance” as determined by a myo-monitor, a transcutaneous electro neural stimulator.
Very recently, a magnetic appliance has been used for treating snoring patients with or without obstructive sleep apnoea [81]. A magnetic appliance has the potential to be more effective than conventional ‘passive’ functional appliances since the magnetic forces prevent the closure by providing direct and continuous mandibular advancement. Long-term evaluation of the treatment results is needed before routine use of the magnetic appliance in apnoea patients.
The Scottish Intercollegiate guidelines Network report (2003) on OSAHS which is also endorsed by the British Thoracic Society concluded that: ‘Intra-oral devices are an appropriate therapy for snorers and for patients with mild OSAHS with normal daytime alertness, intra-oral devices are an appropriate alternative therapy for patients who are unable to tolerate CPAP and the use of intra-oral devices should be monitored following initiation of therapy to allow device adjustment and assessment of OSAHS control and symptoms’ [2].
The advantages of intra-oral appliances therapy are simplicity, reversibility and cost effectiveness. They could also become the treatment of choice for subjects unable to tolerate nasal CPAP or who are poor surgical risks. Most patients readily accept it and it can even supplement other forms of treatment in the few subjects where dental devices alone fail to achieve adequate relief of symptoms.
The disadvantages of intra-oral appliances are: excessive salivation and transient discomfort in the masticators muscles of mastication after awakening are commonly reported during initial use which may inhibit easy acceptance of oral appliances [64,82], but with regular use, and adjustments to fit, these symptoms usually subside. Other research reported that hypersalivation and teeth/gum discomfort are the early side effects but usually decrease if patients are able to persevere with Intra-Oral Devices (IOD) use [83].
Later complications can include TMJ discomfort and changes in occlusion and have been reported as reasons for discontinuing treatment [84]. To prevent these changes the design should use full-arch occlusal coverage to tie all the teeth firmly together [81]. This simply means that the appliance will no longer fit if any individual tooth moves.
However, other research suggests that TMJ dysfunction and occlusal changes are relatively uncommon occurrences, but the long-term risk of such complications requires further clarification [84].
A report exists detailing TMJ problems after a period of 15 months wears; these, however, settled after adjustment of the splint [85].
Efficacy of Oral Appliances: The success rates vary, and different authors’ use differing criteria for its achievement. If reducing the number of apnoeic events by 50% or greater is regarded as adequate, then reported success rates can reach as high as 87% [86]. The most comprehensive review of 20 publications, cataloging the effects of oral appliances on OSAHS [84] showed an improvement in average AHI with a dental appliance. Success was equated with fewer than 10 apnoeic events per hour. Where statistical evidence was provided, the decrease in AHI was always significant (P<0.05), and the mean AHIs before and with treatment were 42.6 and 18.8 respectively, an average reduction of 56%. An improvement in oxygen saturations was also noted, and the time of sleep with oxygen saturation <90% was reduced from 4.4% to 3.1%.
Two recent prospective crossover trials compared mandibular repositioning appliances and continuous positive airway pressure (CPAP) in patients with mild to moderate obstructive sleep apnoea. The success rate with the oral appliance was 55% in both trials, though the improvement in AHI reduction was greater when the continuous positive airway pressure was used [87,88].
A meta-analysis of treatment preference (CPAP and IODs) for patients with mild to moderate OSAHS in three crossover studies produced a significant preference for IODs (OR 9.5, 95% Cl 4 to 21), in spite of their lower nocturnal effectiveness in terms of breathing pauses (-7 per hour, 95% Cl -10 to -5) [88]. However, a later study failed to confirm this [89]. The preference by patients for IODs is important, but no information is available about whether this preference is due to feeling symptomatically better when using IODs or whether the nature of an unobstructive intraoral device makes it more attractive than using an obstructive CPAP device [2].
One group studied the effectiveness of IOD against UPPP use in a parallel group, longitudinal follow-up study, with the last report being made four years after randomisation [57]. The results indicated that 72 out of 95 patients suffering from mild to moderate OSAHS had returned for polysomnography indicating a significant effect size (>1.0 SDs) demonstrably favouring IOD over UPPP regarding an improvement in AHI and desaturation index, but failing to show any significant difference in snoring duration between the treatments.
No formal survey of the cost of devices and services has been carried out for oral appliances. The production costs vary according to whether the services of a dental laboratory are required for custom fitting or whether a prefabricated unit can be adapted the clinician’s own practice. Cephalometric radiographs or other airway studies add to treatment cost when these are necessary.
As yet, reliable figures have not emerged to show whether continuous positive airway pressure (CPAP) or intra-oral devices (IODs) represent better-cost effectiveness and the possibility exists that this factor may be dependent on the severity of OSAHS. The initial expense of many IODs is less than that of a CPAP machine. However, several adjustable IODs are more expensive than CPAP, particularly when the expenses of multiple visits to the surgery for adjustment of the device are necessary [2].
Little data on long-term compliance exists, and the data that does is reliant on patient reports. Based on experience with nasal CPAP, selfreporting often significantly overstate actual objective use [58]. Overall compliance rates vary from (50 to 100%) in different studies, a variance that could be connected to the length of follow–up [84]. The reasons for discontinuing appliance use include the side effects, complications noted above and lack of efficacy.
It is possible for increased nasal resistance to induce sleep-related breathing disorders and disturbed sleep [90-93]. Research has demonstrated that numerous devices, including the nasal-valve dilators (Nozovent; PrevancureAB; Västra Frölunda, Sweden), are able to reduce nasal resistance andimprove nasal breathing [94,95]. The Nozovent device comprises a plastic bar dilating the anterior part of the nose, the valve region, so that the airflow is increased. Its inventors aimed at eliminating snoring and sleep apnoea by the use of the device [96-99]. Their research on the use of the device in a number of studies showed excellent effects on nasal resistance, snoring, and sleep apneas [96-99]. Nevertheless, Metes et al. [95] failed to replicate this and found no effect on snoring, apnoeas, hypopnoeas, or arterial oxygen saturation (Sa, O2) in a small sample of patients, althoughnasal resistance was reduced.
Nozovent is marketed worldwide through pharmacies as a treatment for a number of indications, such as compromised nasal breathing, nocturnal asthma, and dryness of mouth, snoring, and sleep apnoea. Since habitual snoring occurs in 15% of middle-aged adults, with occasional snoring affecting another 30% [100], the market potential for such a device is obviously enormous.
However, further research exists where the results fail to support treatment with the Nozovent nasal dilator in patients suffering from OSAHS [101]. The dilator did have a slight effect on subjective measures of snoring, but no corresponding effect on objectively measures or on the other parameters of sleep-related breathing. Moreover, use of a nasal dilator may possibly delay more appropriate treatment of sleep apnoea if the bed partner feels that snoring has been reduced.
Intra-oral appliances should be employed in the treatment of mild to moderate and severe obstructive sleep apnoea hypopnoea syndrome; treatment of severe cases lies beyond the capacity of intra-oral appliances and would possibly be better treated by CPAP or surgery but the MRA ameliorate the condition.
The answers (1: a, 2: b, 3: a, 4: d).
Download Provisional PDF Here
Article Type: Review Article
Citation: Hosseini HR, Mageet AO (2017) Non-Surgical Therapy of Peripheral Sleep Apnoea Hypopnoea Syndrome: Review Article. Int J Dent Oral Health 3(2): doi http://dx.doi. org/10.16966/2378-7090.227
Copyright: © 2017 Hosseini HR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access