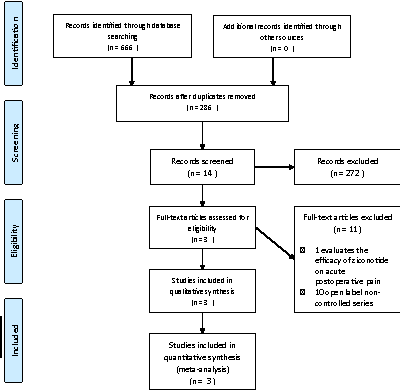
Figure 1: PRISMA Flow Diagram: Literature search and selection

Acevedo JC1 Becerra JE1* Gempeler A2 Caballero J3
1Department of Neuroscience, Faculty of Medicine, Hospital Universitario San Ignacio – Pontificia Universidad Javeriana, Bogotá D.C, Colombia*Corresponding author: Jaime Eduardo Becerra, Department of Neuroscience, Faculty of Medicine, Hospital Universitario San Ignacio – Pontificia Universidad Javeriana, Bogotá D.C, Colombia, Tel: (+57) 1 3173782378; Fax: (+57) 1 7508238; E-mail: jaime10b@gmail.com
Article Type: Review Article
Citation: Acevedo JC, Becerra JE, Gempeler A, and Caballero J (2016) Efficacy and Safety of Intrathecal Ziconotide for the Management of Chronic Pain. A Systematic Review of the Literature and Meta-Analysis Of Randomized, PlaceboControlled Trials. J Clin Anesth Manag 1(5): doi http://dx.doi.org/10.16966/2470-9956.120
Copyright: © 2016 Acevedo JC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Background: Chronic pain is a frequent condition that leads to a significant decline in the quality of life and most patients do not respond to medical treatment. New molecules, such as ziconotide, arise as alternatives in the management of these patients. We meant to determine the efficacy of intrathecal ziconotide in the treatment of refractory chronic pain.
Databases and Data Treatment: As of February 2015, a systematic search of the literature in PubMed/MEDLINE, EMBASE, LILACS, Cochrane (Ovid), American Academy of Pain Medicine, North American Neuromodulation Society, American Pain Society and grey literature was made. We included Clinical Randomized Controlled Trials, which used ziconotide as monotherapy or combined therapy for the treatment of chronic pain. This study follows the Cochrane Collaboration methodology. The main measured outcome is the improvement of pain according to a decrease in the Visual Analogue Scale of Pain Intensity (VASPI) score. Information for other critical outcomes was assessed (improvement of pain in the Category Pain Relief Score [CPRS], Response to Treatment, Safety) with the fixed effects Mantel-Haenzhel model. Subgroup analysis was performed when increased heterogeneity demanded it. The quality of evidence for each study and each outcome was estimated with the GRADE tool of assessment.
Results: The search yielded 666 results, only three of these studies were selected for analysis. All three were double blind, randomized, placebo-controlled trials. The use of ziconotide resulted in adequate pain relieve with VASPI score reduction of over 30% compared to baseline (3 studies, 595 patients, RR 2.04 [CI 95% 1.55-2.7]; p<0.00001) and CPRS improvement (3 studies, 595 patients, RR 4.2 [CI 95% 2.52-7.01]; p<0.00001). The GRADE quality of evidence was moderate due to the risk of blinding bias and indirect comparisons between studies.
Conclusions: Our analysis suggests that intrathecal ziconotide is superior to placebo in the management of refractory chronic pain. The quality of evidence for this outcome was low, and further clarifying research is needed.
Ziconotide, Chronic Pain, Intrathecal Therapy, Adverse Events
It is estimated that approximately 20% of the European population suffer from chronic pain [1]. As population gets older and the survival rates of diseases that can cause this condition improve, chronic pain rates increases [2]. Due to its impact on quality of life, chronic pain contributes to disease burden3. However, therapeutic options to manage chronic neuropathic pain is limited and, more than 60% of affected patients express dissatisfaction with treatment or refer persistent pain for many years [1-3].
Historically, different types of treatment have been used; ranging from analgesics (such as acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs) and opiates), up to coadjuvant therapy with antineuropathics and antiepileptic drugs (such as carbamazepine or pregabaline) [4,5].
Ziconotide, a conopeptide that selectively blocks voltage-dependent type N calcium channels6, seems to be a promising alternative for these patients. This drug has been used as experimental therapy for patients with neoplasic diseases, AIDS and neuropathic pain of non-neoplastic origin since 2016. Ziconotide has shown to be effective in controlling neuropathic and somatic chronic pain, including that refractory to opioid therapy [2,6] it has been approved as first-line treatment for intrathecal management of pain [7]. Even though, its use in clinical practice is still limited. It has been related with important adverse events such as acute cardiovascular toxicity [8], intractable delirium [9] and risk of suicide [10]. Nevertheless there is not strong evidence to support these findings; the majority of them come from uncontrolled studies and case reports [6,3,11].
Due to the potential clinical effect on chronic pain control, that ziconotide could have, it is important to summarize the existing evidence with the aim of defining its real effects and to balance the benefit and harms of its use. In this systematic review we aim to assess the comparative effect and safety of ziconotide versus morphine when are used intratecally in patients with chronic pain who have not responded to conventional treatment. We also aim to test the hypothesis that adverse events related to ziconotide could be associated to its administration at high doses, therefore we include comparative studies that assess differential doses of intratecal ziconotide.
We performed a systematic search in different databases with the following limits:
Period 1980 to February 2015
Databases: Medline, Cochrane, EMBASE, LILACS, Ovid (Books@ Ovid Journals@Ovid Full Text, EBM Reviews - ACP Journal Club, EBM Reviews - Cochrane Central Register of Controlled Trials, EBM Reviews - Cochrane Database of Systematic Reviews, EBM Reviews - Cochrane Methodology Register, EBM Reviews - Database of Abstracts of Reviews of Effects, EBM Reviews - Health Technology Assessment, EBM Reviews - NHS Economic Evaluation Database, Global, Inspec, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE (R), Ovid MEDLINE(R) Daily Update, Ovid OLDMEDLINE(R) )
Additionally, we performed a manual search of grey literature in intrathecal therapy- related congresses and meetings of scientific associations. We searched for abstracts presented to the American Academy of Pain Medicine, the North American Neuromodulation Society and the American Society of Pain. Three authors conducted the search independently; studies selected for analysis were the same among the authors.
We report this systematic review according to the PRISMA statement [12].
Two authors (AG-JB) extracted relevant data. We registered information about the number of patients, intrathecal medication, administered dosage, efficacy outcome, results (according to pain or quality of life improvement – VASPI, McGill, etc.) and safety data (adverse events) for each study.
The main outcome was:
The secondary outcomes were:
One author (JB) evaluated the methodological quality of the selected papers using the risk of bias assessment tool proposed by the Cochrane Collaboration [13]. Additionally the overall quality of evidence for each critical outcome using the GRADE quality assessment criteria [14].
We analyzed the data reported in trials for the aforementioned outcomes. The Mantel-Haenzhel method was used to obtain estimates of the Relative Risk (RR) and Risk Difference (RD). We established our type I error in α=0.05 and reported a Confidence Interval of 95% (CI 95%) with every result. We reported the Number Necessary to Treat (NNT) for statistically significant results. All meta-analyses were conducted using the fixed effects model. For meta-analyses, we used the software supplied by the Cochrane Collaboration, RevMan 5.2.
We examined heterogeneity and its impact amongst trials with the I² value [13]. We considered an I² value > 50% as substantial heterogeneity amongst trials. We searched and described the possible causes (differences in trials quality, number of participants, intervention regime, and outcome analysis). When heterogeneity could not be explained or eliminated, we analyzed the data with the random effects model.
The ziconotide high dose vs. low dose subgroup was reported in one trial. The ziconotide vs. intrathecal morphine subgroup is not reported because all trials had a pre-infusion phase (in which all intrathecal opioids were suspended) and the dosage of oral opioids that replaced them was comparable between the intrathecal ziconotide and placebo groups.
The literature search yielded 286 references. Out of these, 14 were preselected, but only three Rauck et al., Wallace et al., Staats et al. met the inclusion criteria and were used for meta-analysis [15-17]. The remaining 11 were excluded, 10 were non-controlled studies such as case series, and one randomized controlled trial that was excluded because it evaluated acute postoperative pain [18-28] (Figure 1).

Figure 1: PRISMA Flow Diagram: Literature search and selection
All studies that describe the effectiveness of ziconotide [15-17] included 595 patients and reported on main and secondary outcomes (Table 1).
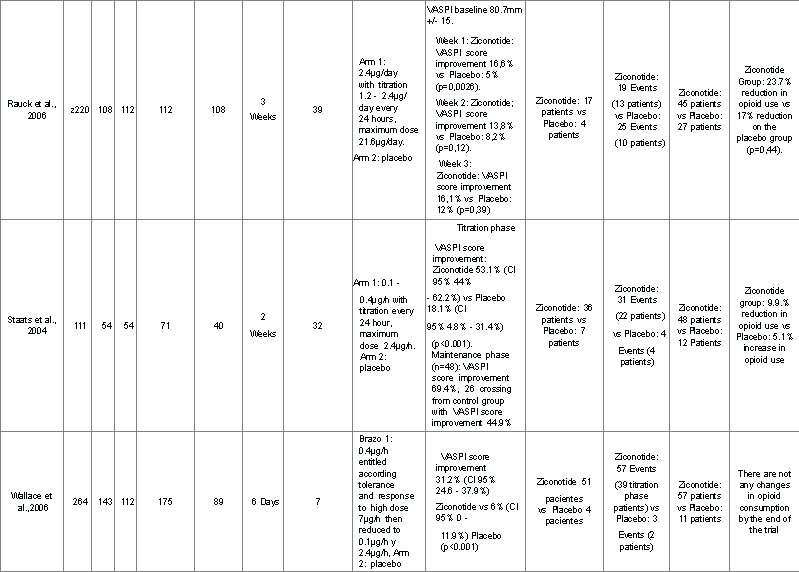
Table 1. Efficacy of the Use of Intrathecal Ziconotide Summary of Clinical Data
We found all the included trials to have low risk of bias for randomization, allocation, concealment, complete data at follow-up and intention to treat, with the adequate standardization of observation periods and clear outcomes. However, the authors did not report adequately the method nor the technique of patient randomization and allocation. In addition, only one of the trials Rauck et al. [18] held blinding until completion of the trial, whereas the other two had a crossover phase where blinding was lost and patients from the control group were administered the experimental medication (ziconotide). Therefore, we considered that in their second phase these trials have a high risk of blinding bias and thus were classified as having a “Not Clear” risk of bias for blinding (Table 2).
Detailed descriptions of each of the selected trials regarding population, intervention and outcomes are presented in Table 1. Rauck et al. [18] Prospective double blinded, randomized, controlled trial of two
arms with 220 patients admitted in 39 international centers, 112 are allocated to receive ziconotide and 108 placebo.It evaluates the efficacy and safety ofintrathecal ziconotide with titration of dosage for 3 weeks. Evaluated outcomes were: Pain according to VASPI score, CPRS and McGill scale; quality of life and frequency of adverse events. Loss to follow-up was 8% for the ziconotide arm vs. 4% for the placebo arm. On the first week of treatment there is an improvement of VASPI score of 16.6% for the ziconotide group vs.5% in the placebo group (p=0.0026). On the second week, improvement of VASPI score is 13.8% for ziconotide vs 8.2% for placebo (p=0.12). On the third week the improvement of VASPI score is 14.7% for ziconotide vs 7.2%for placebo (p=0.036). By the end of trial, there is improvement in the satisfaction with the therapy in 28% of patients who received ziconoide vs.12.1% in the patients who received placebo. 17 patients in the ziconotide group and 4 patients in the placebo group achieve improvement according toCPRS (patients who ranked in the upper categories of the scale - Great Relief and Complete Relief). This difference however is not statistically significant (p=0.0596). Reduction in McGill pain scale score is statistically significant(p=0.026). Quality of life measured by the TOPS questionnaire does not demonstrate statistically significant changes (p=0.1837). There is a 23.7%reduction of opioid use in the ziconotide group vs 17.3% in the placebo group(p=0.44) but this difference is not statistically significant. Serious adverse events were dizziness, confusion, ataxia and abnormal gait. These were equally common for both groups (11.6% ziconotide vs 9.3% placebo, p=0.57). Staats 2004: Prospective double blinded, randomized, controlled trial of two arms with 111 patients with AIDS or cancer and VASPI score >50mm are admitted in 32 international centers, 71 are allocated to receive ziconotide and 40 to placebo. Randomization and allocation is done in a 2:1 ratio favoring the ziconotide group. The trial is divided in several phases; the first is the screening phase, which includes removal of intrathecal opioids and the implantation an internal or external intrathecal infusion pump to patients for a total infusion period of two weeks. In the second phase, or titration phase, patients receive ziconotide or placebo for 5 days and those with improvement of pain continue into a thirdstage or maintenance phase for another 5 days. Patients who did not respondto treatment are crossed over to the opposite group for 5 to 6 days loosing blinding. Response to treatment is defined if there is improvement of VASPIscore >30% from baseline without increasing the dosage of opioids. Outcome measures were improvement of pain according to reduction of VASPI, CPRS, Wisconsin Brief Pain inventory (WBPI) and Karnofsky Performance Status Score (KPSS). In the titration phase, there is improvement of VASPI score of 53.1% (CI 95% 44 – 62.2%) with the use of ziconotide vs 18.1% (CI 95% 4.8% - 31.4%) with placebo (p<0.001). In the maintenance phase (n=48) there is a reduction of VASPI score in 69.4% from baseline. 26 patients who crossed over from the control group to the ziconotide group achieve a VASPIscore reduction of 44.9% from baseline. Adverse events are registered according to the number of days from the starting dose of ziconotide and their time of onset, mean dosage at onset and mean cumulative dosage at onset. The most frequent adverse events are dizziness (36 events, mean days toonset 2.5 ± 0.2 days, mean dosage at onset 27.3 μg ± 5), nausea (34events, mean days to onset 2.44 ± 0.3 days, mean dose at onset 0.89 μg/h ± 0.2, mean cumulative dose at onset 34.2 μg ±1), nystagmus (33 events,mean days to onset 2.76 ± 0.2 days, mean dose at onset 1.45 μg/h ± 0.6 and mean cumulative dose at onset 51.3 μg ±19.8), somnolence (17 events,mean days to onset 2.47 ± 0.3 days, mean dose at onset 0.84 μg/h ± 0.2,mean cumulative dose at onset 30.8 μg ± 9.3) and confusion (15 events,mean days to onset 3 ± 0.4 days, mean dose at onset 0.62 μg/h ± 0.1 and cumulative dose at onset 39.8 μg ± 14). Wallace 2006: Prospective double blinded, randomized, controlled trial of two arms which includes 257 patients, 169 allocated to the ziconotide groupand 86 to the placebo group in a 2:1 ratio. These patients had chronic refractory pain, non-neoplasic in origin, refractory to conventional treatment with a VASPI score >50mm. These patients receive treatment for 6 days with a starting dose of ziconotide of 0.4 µg/h titrated upwards until the maximum dose tolerated is achieved (around 7 µg/h). Later, during recruitment the authors had to decrease the starting dose to 0.1 µg/h with a máximum dose of 2.4 µg/h due to adverse events and loss of patients. Reduction of VASPI score from baseline is 31.2% for the ziconotide group vs. 6% for the placebo group (p<0.001). Adverse events are reported on the titration phase for the ziconotide group (gait abnormalities, dyplopia, dizziness, nausea, vomiting and urinary retention).
Pain relief according to VASPI score: ziconotide vs. placebo
Pain relief, according to reduction in VASPI score depending on administration of ziconotide vs. placebo is reported in the three included trials. Rauck et al.[2] reported a 14.7% reduction in VASPI scores in the ziconotide group and 7.2% in the placebo group (p=0.036) with a baseline VASPI score mean of 80.7 mm. Staats et al. [19], based on an Intention to Treat (ITT) analysis, reported a 51.4% reduction in VASPI score for the ziconotide group vs 18.1% for the placebo group (CI 95% , 17.3% - 49.4%) (p<0.001) with baseline VASPI score means of 73.6 mm and 77.9 mm, respectively (p=0.18). Wallace et al. 2006 reported a 31.2% reduction in VASPI score (CI 95%, 24.6%- 37.9%) for the ziconotide group vs 6% (CI 95% 0-11.9%) for the placebo group (p<0.001), baseline VASPI score means were 80.1mm for the ziconotide group and 76.9 mm for the placebo group (p=0.029).
According to GRADE tool, the quality of this evidence is moderate due to risk of bias by indirect comparisons between different populations amongst the three trials (Table 2).
Pain relief according to the CPRS score due to the administration of ziconotide or placebo is reported in the three trials as well. Pain relief was considered when patients were classified as having “Great Relief ” or “Complete Relief ” in the CPRS scores.The meta-analysis included all the trials Figure. 1 and demonstrated a statistically significant probability of being in the categories of pain relief of the CPRS score when ziconotide was administered (3 trials, 595 patients, RR 4.2 [IC 95% 2.52 - 7.01]; RD 0.21 [CI 95% 0.16 – 0.26]; I²: 0%; p<0.00001; NNT 5 [IC 95% 3.5-5.9]). Quality of evidence based on the Grade assessment tool is moderate due to risk of bias by indirect comparisons between different populations amongst the three trials (Table 2).
Outcome |
N° Patients (Trials) |
Quality of Evidence (GRADE) |
Relative risk (95% CI) |
Reduction (VASPI) Score |
595 |
⊕⊕⊕⊝ |
RR unreported |
Improvement CPRS (Category Pain Relief Scale) |
595 |
⊕⊕⊕⊝ |
RR 4.2 (2.52 - 7.01) |
Analgesic response |
595 |
⊕⊕⊕⊝ |
RR 2.04 (1.55 - 2.7) |
Serious adverse events (AEs) |
595 |
⊕⊕⊝⊝ |
RR 2.3 (1.54 - 3.43) |
Opioid use |
595 |
⊕⊕⊕⊝ |
RR unreported |
|
|||
CI: confidence interval; RR: Relative risk. |
|||
Levels of evidence according to the GRADE Working Group |
|||
Table 2. Quality of Evidence (GRADE)
Response to treatment with ziconotide administration or placebo is reported on all three trials. The definition for response to treatment was the same amongst trials and is defined as reduction of >30% in the VASPI score without an opioid dose increase or opioid change.The meta-analysis included all trials (Figure 2) and demonstrated a statistically significant improvement in response to treatment with the administration of ziconotide (3 trials, 595 patients, RR 2.04 [IC 95% 1.55-2.7]; RD 0.21 [IC 95% 0.14-0.29]; I²:11%; p<0.00001; NNT 5 [IC 95% 3.6-7.4]). The quality of evidence based on the GRADE assessment tool is moderate due to risk of blinding bias in the crossover phase in two of the three trials (Table 2).
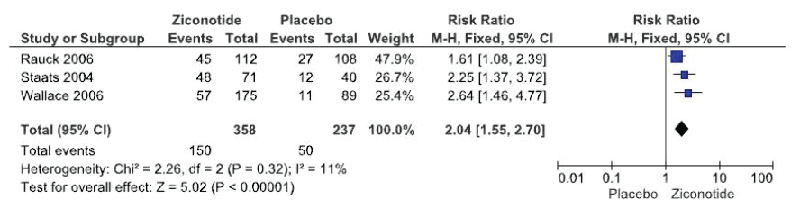
Figure 2: Ziconotide vs placebo analgesic response (>30% reduction of VASPI scores). ITT analysis (Forest Plot)
All studies reported on the incidence of adverse events in a similar way. They all showed a tendency for higher incidence of adverse events in the ziconotide group, with over 90% of patients reporting any adverse event (Rauck et al.[2]: 92.9% ziconotide vs 82.4% placebo; Staats 2004: 97.2%
ziconotide vs 72.5% placebo; Wallace et al.[20] 2006: 94.6% ziconotide vs 69.6% placebo). However, the presence of serious adverse events, (described across all trials as confusion, nystagmus, ataxia or urinary retention) was less frequent.
The meta-analysis of the three trials Figure. 3 had to be done by subgroup analysis due to the great heterogeneity of the trials, with a funnel plot that can suggest publication bias due to wide data dispersion Figure. 4. This heterogeneity is explained by differences in randomization in one of the trials Rauck et al. [18] which is done in a 1:1 ratio (ziconotide and placebo on equal proportions) compared to the other two trials in which randomization was done in a 2:1 ratio, favoring ziconotide.
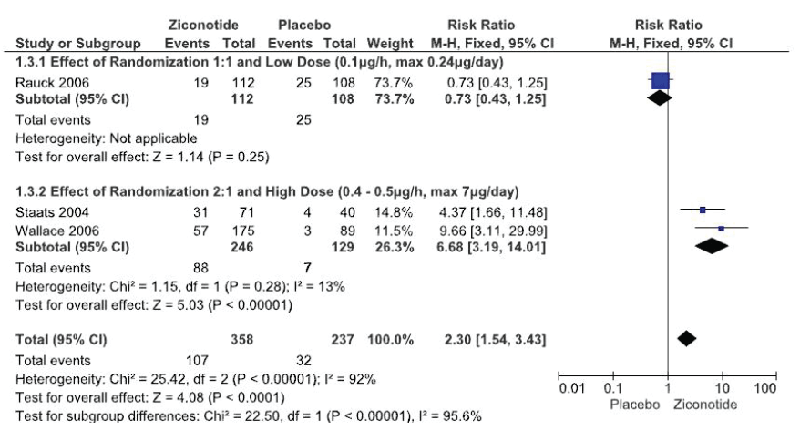
Figure 3: Serious Adverse Events. ITT Analysis (Forest Plot)
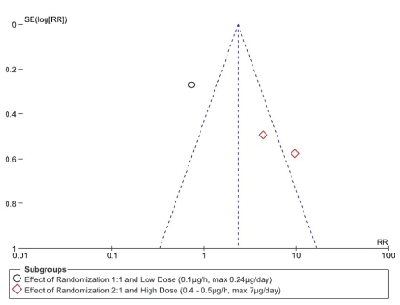
Figure 4: Funnel plot of comparison: Serious Adverse Events (ITT Analysis)
The first subgroup Rauck et al. [18] did not demonstrate a statistically significant difference in the incidence of serious adverse events with ziconotide administration compared to placebo (1 trial, 220 patients, RR 0.73 [IC 95% 0.4 –1.25]; RD -0.6 [IC 95% -0.17–0.04]; I²: does not apply; p=0.25).
The second subgroup (Staats et al. [29] and Wallace et al. [30] demonstrated a statistically significant increase in the risk of presenting a serious adverse event with ziconotide administration compared to placebo (2 trials, 375 patients, RR 6.68 [IC 95% 3.19 – 14.01]; RD 0.31 [IC 95% 0.23-0.38]; I²: 0%; p<0.0001; Number Needed to Harm (NNH) 4 [IC 95% 2.7 – 4.3]). However, the quality of this evidence is low due to the great heterogeneity of these trials (Table 2).
Opioid consumption was reported in all included trials. Rauck et al.[18] reported a mean reduction of 23.7% in weekly opioid consumption compared to a 17.3% reduction in the placebo group, although it was not statistically significant (p=0.44). This reduction ranged from 2101 mg (morphine equivalents) prior to treatment to 1524 mg after treatment for the ziconotide group, and from 1876 mg prior to treatment to 1453 mg after treatment for the placebo group.
Staats et al. [19] reported a reduction in opioid consumption of 9.9% for the ziconotide group with a 5.1% increase for the placebo group. Wallace et al.[20] reported that opioid consumption did not change for either group.
The quality of evidence is moderate due to risk of blinding bias in the crossover phase in two of the three trials.
Intrathecal ziconotide has been approved as an alternative for the management of severe chronic pain in patients who are intolerant or refractory to other types of analgesic therapy (antineuropathics, opioids, etc.). Furthermore, its effectiveness has been proven by three separated randomized, controlled trials using different types of population. In addition, there are not any established guidelines about the appropriate dosage for pain control without the onset of adverse events. At present, even though there is evidence in the literature [29-34] that supports ziconotide use and dosage, there does not exist a systematic review that summarizes its effectiveness in the management of chronic pain. Moreover, it does not exist either a meta-analysis that quantifies such effectiveness.
This is the first study in which a systematic review of the literature and meta- analysis of prospective randomized, controlled, clinical trials comparing the efficacy of ziconotide vs. placebo for the treatment of severe chronic pain is performed.
There are multiple measures available to assess pain in adult chronic pain populations. To evaluate the multiple dimensions of acute and chronic pain, a number of valid and reliable questionnaires are available.
The Visual Analog Scale for Pain Intensity (VASPI) is a unidimensional single-item scale that provides an estimate of patients’ pain intensity. The VASPI is a continuous scale comprised of a horizontal (HVAS) or vertical (VVAS) line, usually 10 centimeters (100 mm) in length, anchored by 2 verbal descriptors, one for each symptom extreme. For pain intensity, the scale is most commonly anchored by “no pain” (score of 0) and “pain as bad as it could be”or “worst imaginable pain” (score of 100 [100-mm scale]). A higher score indicates greater pain intensity. Based on the distribution of pain VAS scores in post surgical patients who described their postoperative pain intensity as none, mild, moderate, or severe, the following cut points on the VASPI have been recommended: no pain (0-4 mm), mild pain (5-44 mm), moderate pain (45-74 mm), and severe pain (75-100 mm). Normative values are not available [35]. The Categorical Pain Relief Scale is a unidimensional scale in which patients report their pain intensity and relief according to a 5 categorical scale. It is limited in nature due to inferior sensitivity in comparison with numeric rating scales or the VAS. In-house & Adler (1975) has used both a visual analogue (VAS) and verbal rating scale (VRS) in a double-blind, complete crossover study of analgesics in pathological pain and have found a high correlation between the two scales, although the rating scale in their hands produced more highly significant results in terms of drug differences. They interpreted this to imply that verbal ratings tend to distort by forcing patients to choose a category and that visual analogue scales more closely assessed the patient’s experience [36].
The VASPI score is a quantitative scale designed to minimize the subjective variability of pain. It allows a reproducible and comparable measurement of pain in the clinical environment. The ziconotide use has proven to be superior to placebo in reducing VASPI scores [15- 18,20,22,23,37-40], explained by its mechanism of action which consists on direct and selective blockage of N type calcium channels already established by preclinical trials [41-46].
On preclinical experiments, ziconotide has shown that it can be used for neuropathic pain as well as for somatic pain [19]. This feature has been demonstrated in animal models of central sensitization like the paw flinching response in the late phase of the formalin test in mice [20]. The results obtained in our review are consistent with previous studies [7,15-17,21-23,30,38,47]. The low variability of the results regarding this outcome amongst trials suggests that VASPI score reduction is consistent and reliable as a measure of treatment effectiveness. Among the limitations of the analysis of this outcome, we found that the trial’s authors did not specified standard deviations nor the VASPI score reduction range.
Pain relief according to the CPRS score demonstrated an important and statistically significant difference in favor of administering ziconotide over placebo. In the measurement of this outcome figure 1), all three trials were quite homogenous, although the statistical power of each trial on the effect of the intervention has to be weighted. In 2 of the 3 studies (Staats et al. [19] and Wallace et al.[20]), randomization was performed in a 2:1 ratio in favor of the ziconotide group. The authors mentioned that this was done to readily identify the appearance of adverse events and determine the safety dose of ziconotide. However, the trials statistical power is reduced to 0.925 while the established standard power in clinical trials is 0.95. This occurs because this type of randomization allocates 66% of patients from the sample in the ziconotide arm 48, therefore, Staats’ et al. and Wallace et al.[20] trials can diminish the real effect of ziconotide. However, we consider the CPRS score as a reliable measure of the effectiveness of ziconotide with an NNT of 5 to obtain benefit from the medication.
The comparative power of our study is limited because the selected trials are the only ones in the literature that have as a secondary outcome relief in pain according to CPRS but it leaves a precedent in the literature as point of reference for future trials.
In the present meta-analysis figure. 2 we demonstrate a statistically significant difference regarding analgesic response in favor of intrathecal ziconotide. The heterogeneity of the trials is low which makes analysis easier and confers security and consistency in the results. The NNT is 5 to achieve an analgesic response.
Our results are consistent with prior systematic reviews and descriptive case series [7,21,22, 25,27,30, 31,32, 34,38, 47,49,50] which demonstrate an adequate analgesic response in patients who receive intrathecal ziconotide. This response to pain is attributed to its mechanism of action as a selective type N channel blocker, limiting the neuronal depolarization and propagation of the pain stimulus. However, this outcome is analyzed in three different populations with specific and controlled clinical conditions. A study, which analyzes the effectiveness of ziconotide in an outpatient clinic environment, with non-controlled conditions and its interaction with other molecules is still lacking.
Safety: The serious adverse events in the analyzed trials were ataxia, nystagmus, somnolence, confusion and urinary retention. Our metaanalysis was done by subgroup analysis due to the great heterogeneity of the results. This is also explained by protocol differences of dose titration in two of the three trials Staats et al.[19] and Wallace et al.[20].
Our results are consistent with previous safety trials [6,11,18,21,22,29,30,43,51-53] Nevertheless, there are also case reports in the literature about the onset of infrequent but serious adverse events with devastating consequences such as psychiatric disorders with risk of suicide [53-55] cardiovascular toxicity [8] or dyskinesia [56] which are not reported in the three trials selected for our meta-analysis.
The quality of the evidence (Table 3) in this regard is low because of the great heterogeneity of results and the non-comparable populations, which in some cases could have been more prone to presenting adverse events, such as the Staats et al.[19] population.
All three trials had a pre-infusion phase in which all intrathecal medications were withdrawn to included patients, achieving pain control with systemic or oral opioids. However, in spite of pain relief achieved with ziconotide administration, none of the trials demonstrated an opioid use reduction, although Staats et al.[19] reported such a tendency, it was not statistically significant.
Unlike previous systematic reviews which are not specific [29-34], our study is the only systematic review, which includes exclusively randomized, double-blind, controlled clinical trials with the objective of quantifying the effect of ziconotide with the use of a meta-analysis. This restriction limits the number of included studies and can affect the statistical power of the meta- analysis. Nevertheless, these restrictions in our study allow us to select trials with evidence level I and II making our analysis less prone to bias.
To avoid publication bias, we performed an extensive search of the literature, including gray literature and abstracts of papers not yet published but presented to the North American pain societies. With this search we found there is only one meta-analysis, which sought to answer the question about the efficacy and safety of ziconotide use for chronic pain but there is no publication of this trial in any database [57]. It is likely that it was published in some non-indexed source of information to which we do not have access.
The quality of evidence for most outcomes was moderate because all trials incurred in at least one limitation or bias, which reduced their epidemiological quality according to the GRADE tool. This is more notorious in the safety outcome where the great heterogeneity of the results given the differences on initial dosing and the effect of randomization, which favored ziconotide, reduced the statistical power of the trial and maximized the frequency of adverse events.
We can also observe that there are no studies that compare the efficacy of intrathecal ziconotide to intrathecal morphine, which is considered the opioid of choice for intrathecal infusion. Furthermore, no long term randomized, double blind, placebo-controlled trials that assess ziconotide effectiveness under daily clinical conditions exists.
Our analysis demonstrated that intrathecal ziconotide is superior to placebo in the management of refractory chronic pain with adequate pain control from different types of patients (AIDS, cancer, neuropathic pain). However, this drug is associated with an increased number of adverse effects such as somnolence, nystagmus, ataxia, or psychiatric disorders. The quality of evidence for this outcome is low which is why further research may be warranted.
Maria Ximena Rojas,Doctor in Public Healthcare and Methodology of Biomedical Research Master in Clinical Epidemiology, Associate Professor.
All authors have worked in preparing the document, discussed the results and commented on the manuscript accepting this as a final form.
This work has been funded entirely by the Hospital Universitario San Ignacio and the Faculty of Medicine of the Pontificia Universidad Javeriana.
We state that we do not have any conflicts of interest regarding any pharmaceutical company, nor have we received any benefit from our work.
Download Provisional pdf here
All Sci Forschen Journals are Open Access