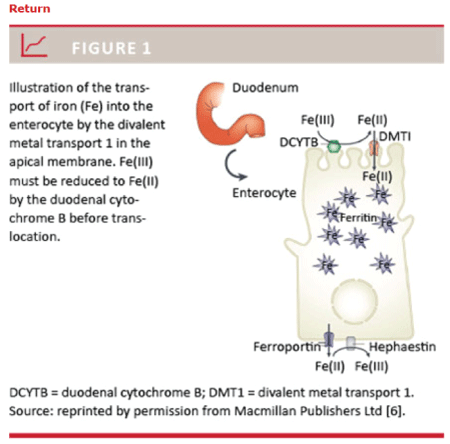
Figure 1: Fe3+ must be reduced to Fe2+ by the duodenal cytochrome B before translocation can occur
Rondinelli MB* Di Bartolomei A De Rosa A Pierelli L
Department of Transfusion Medicine, San Camillo Forlanini Hospital, Rome, Italy*Corresponding author: Rondinelli MB, Department of Transfusion Medicine, San Camillo Forlanini Hospital, Rome, Italy, E-mail: bertini@baldaccilab.com
Introduction: In an oral iron absorption test (OIAT), the rise in plasma iron concentration after oral ingestion of iron is a measure of intestinal iron absorption. We describe results of the OIAT using a new co-processed compound between Ferrous By sglicinate Chelate and Alginic Acid named FERALGINE® in capsules.
Methods: The study included all patients with IDA who had an OIAT performed at the Department of Transfusion Medicine of San Camillo Forlanini Hospital in Rome, Italy from June 2016 to September 2016 using two capsules containing each one 30 mg of elemental iron from FERALGINE®. A venous blood sample was drawn before and then 120 minutes after the intake of 2 capsules (60 mg of elemental iron) of FERALGINE® (TECNOFER® PLUS).
Results: The patient characteristics were similar in terms of age, hemoglobin, ferritin and plasma iron. The fasting baseline plasma iron concentration was median 11.21 mg/dl. The maximum plasma iron concentration increase from baseline after 120 minutes of oral intake of FERALGINE® was median 111.0 mg/dl (T Students Test p<0.00001).
Conclusion: OIAT performed with FERALGINE® capsules results in a statistically significant increase of plasma iron concentration after 120 minutes (p<0.00001) showing an excellent and quick oral bioavailability of the new patent-pending compound FERALGINE® in IDA patients.
Iron Deficiency anemia (IDA) is still considered the most common nutrition deficiency worldwide [1-3]. Decreased Iron absorption, deficient iron intake, increased iron loss and request can result in (IDA) [3]. Oral iron therapy still remains the first-line approach for treating IDA [4]. It is safer, more cost-effective, and convenient when compared with parenteral iron therapy: unfortunately, occurrence of gastro-intestinal adverse events during oral iron therapy could represent a limit for patien’s domiciliary compliance [5,6]. Meals could reduce these iron adverse effects, but, unfortunately, iron absorption may decrease by 40% [5,6]. A reduced absorption of dietary iron and iron tablets occurred also during PPIs (Proton Pump Inhibitors) treatment and during events inducing gastric acid hypo secretion [5]. Since the limits of oral iron therapy is the domiciliary compliance of the IDA patients to the treatment because of tolerability (gastro-intestinal adverse effects) a lot of attempts have been done with different iron salts [5,6]. Iron (Fe) is an essential trace mineral that is naturally present in many foods, added in some foods and available as a dietary supplement. Iron exists in a wide range of oxidation states, but mainly as Fe2+ (ferrous) and Fe3+ (ferric) [7]. After oral ingestion of iron, the intestinal absorption of iron occurs in the duodenum and the proximal jejunum (Figure 1) [8]. Fe2+ is actively transported into the enterocytes by the divalent metal transporter 1 (DMT1), and the expression of DMT1 is regulated by the intracellular level of iron. Fe3+ must be reduced to Fe2+ by the duodenal cytochrome B before translocation can occur (Figure 1) [8].

Figure 1: Fe3+ must be reduced to Fe2+ by the duodenal cytochrome B before translocation can occur
This is the reason why today is still generally believed that oral ferrous ion formulations have advantages over ferric formulations and Ferrous iron formulations are recommended by The International Nutritional Anemia Consultive Group (INACG), the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) for treatment and prevention of iron deficiency [9] is a new source of iron consisting. In order to decrease to lesser extent gastrointestinal adverese events and to ameliorate patient’s domiciliary compliance, a new source of iron, Ferrous Bysglicinate Chelate, has been chemically and clinically developed [10- 21]. In Ferrous Bysglicinate Chelate one molecule of ferrous iron is bound (chelated) by two molecules of glycine resulting in two heterocyclic rings [10]. After oral administration Ferrous bysglicinate Chelate is absorbed intact, like “chelate”, by the mucosal cells of the intestine: only after duodenum absorption this particular source of iron is hydrolized into its glycine components and iron [11]. This particular pharmacokinetic profile of Ferrous Bysglicinate Chelate is confirmed by a lot of clinical trials showing clinical bioequivalence between this source of iron at low dosage and ferrum sulphate at high dosage (1 to 4 ratio) following oral administration [13-16]. Unfortunately, during oral treatment with Ferrous Bysglicinate Chelate some gastro-intestinal adverse effects occurred, expecially with dosage over 14mg of elemental iron, representing a limit, also is at a lesser extent comparing to other iron salts, for patien’s domiciliary compliance to oral iron therapy [12]. Another limit of Ferrous Bysglicinate Chelate could be represented by “the iron taste” of the oral preparation that could worsened patient’s domiciliary compliance. To ameliorate even more the bioavailability, taste and tolerability of Ferrous Bysglicinate Chelate has been recently developed a new oral iron source named FERALGINE®: it is a compound produced by “spray-drying” methodology resulting in a “co-processed” compound between Ferrous Bysglicinate Chelate and Alginic Acid, two well-Known substances defined G.R.A.S. (Generally Recognized As Safe) by F.D.A. (Food and Drug Administration) [10]. In patients affected by G.E.R.D. (Gastro-Esophageal - Reflux - Disease), gastric hyperacidity and gastric pyrosis the use of Alginic Acid salts is well Known [22,23]. Applying spray-drying technology to the two well known substances a new patent-pending compound with a 1 to 1 ratio and with a very attractive profile in terms of bioavailability, safety and taste, has been developed [10] (Figure 2).
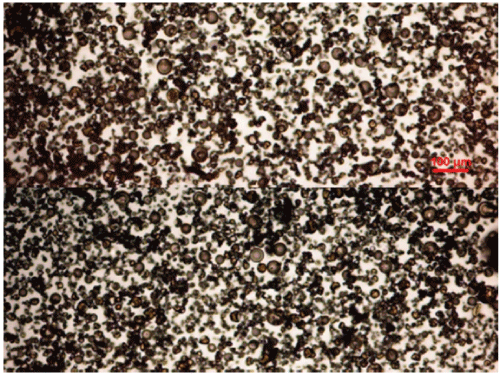
Figure 2: Photograph of the Feralgine powder obtained by spray-drying iron bisglycinate chelate and alginic acid (Protanal® LFR) in a ratio of 1:1. The photograph was taken with an optical microscope LEITZ DIAPLAN with a NIKON DIGITAL SIGHT DS-U1 camera
The oral iron absorption test (OIAT) is a method used to evaluate intestinal iron absorption in humans [9]. In this test, the plasma iron concentration increases after oral ingestion of iron is used as a measure of intestinal iron absorption [24,25]. OIAT has been performed with a lot of iron salts resulting in different bioavailability related not only to the patient’s conditions (mal absorption) but also to the oral iron salts used [9,26-29]. In the present study, we describe our results relating to the OIAT performed before and two hours (120 minutes) after 2 FERALGINE capsules (30 + 30 mg = 60 mg of elemental iron) oral administration in 14 adults consecutive patients affected by IDA.
This is an open, uncontrolled, prospective clinical trial including 14 patients with Iron Deficiency Anemia (IDA), who presented to Department of transfusion Medicine, San Camillo Forlanini Hospital, Rome between July and September 2016. All the patients (8 females and 6 males) completed the study, medium age of the patients was 55,28 ± 8,17. The exclusion criteria were the following: anemia other than IDA, malignancy, mal absorption syndrome, history of gastrointestinal surgery, pregnancy and use of any systemic medication that might affect the iron absorption. An Oral Iron Absorption Test (OIAT) has been performed. In OIAT fasting serum level is compared with a second serum iron level obtained two hours (120 minutes) following oral ingestion of 2 TECNOFER® PLUS capsules of 595 mg (30 mg of elemental iron each one belonging to FERALGINE® - total elemental iron content 60 mg) along with water. A medium serum iron increase of 100 mg/dl after three hours indicated that oral iron absorption is usually adequate. The patient has been informed about the test. Participants iron level was measured at baseline and 2 hours (120 minutes) after receiving two oral iron capsules. After overnight fasting at 8.00 am 60 mg of elemental iron belonging to FERALGINE®- TECNOFE® PLUS: Laboratori Baldacci SpA) were given orally. Serum iron was measured just before and 2 hours after taking iron. The patients were not allowed to ingest food before the last blood sample was drawn at 10.00 am. The difference between two measurements was noted. For the statistics analysis of the study SPSS 20 software (SPSS Inc, Chicago, IL, USA) was used. One sample T- Students Test with 26 degrees of freedom was calculated between serum iron level before and 2 hours after oral iron administration. One side p value <0.05 was considered statistically significant. All the adverse events to the oral iron treatment have been annoted during the trial.
14 patients have been enrolled in the study. At enrollment time (T0– baseline) every patient has been affected by IDA (mean Hb value: 9,7 g/dl ± 0,7) and ferritin medium levels was 8,23 ± 3,29 µg/l. Clinical and initial laboratory findings of the study populations are summarized on table 1.
| Pz | Age | Sex | Hb (g/dl) | Ferritin (µg/l) | Plasma Iron (µg/dl) |
| 1 | 56 | F | 10,5 | 3 | 3 |
| 2 | 62 | M | 11 | 10 | 40 |
| 3 | 65 | M | 10,2 | 5 | 24 |
| 4 | 48 | F | 9,7 | 12 | 6 |
| 5 | 45 | F | 9 | 4 | 22 |
| 6 | 51 | M | 10,2 | 11 | 4 |
| 7 | 50 | F | 9,7 | 12 | 5 |
| 8 | 67 | M | 9,2 | 6 | 11 |
| 9 | 65 | M | 9 | 11 | 10 |
| 10 | 46 | F | 10,7 | 10 | 3 |
| 11 | 67 | M | 8,9 | 5 | 11 |
| 12 | 52 | F | 10,1 | 7 | 10 |
| 13 | 51 | F | 8,9 | 11 | 6 |
| 14 | 49 | F | 8,7 | 4 | 2 |
| 55,28 | ----- | 9,7 | 8,23 | 11,21 |
Table 1: Plasma iron statistically significant increased after taking FERALGINE® in fasting condition (after 2 hours) (T2) from 11.21 ± 10.66 to 111.00 ± 51.56 µg/dl (Student’s T Test for paired samples p<0.00001).
Plasma iron statistically significant increased after taking FERALGINE® in fasting condition (after 2 hours) (T2) from 11.21 ± 10.66 to 111.00 ± 51.56 µg/dl (Student’s T Test for paired samples p<0.00001) (see figure 3).
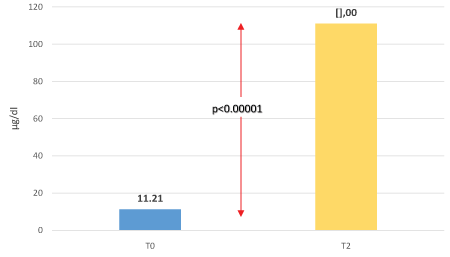
Figure 3: Oral Iron Absorption Test (OIAT): plasma iron before (T0) and after 2 hours (T2) from 60 mg of elemental iron containing in FERALGINE® (p<0.00001 one sample T-Student’s Test).
The principal implication of our observations is that an OIAT performed by using FERALGINE®, a new patent-pending spray drying “co-processed” compound between Ferrous Bysglicinate Chelate and Alginic Acid, results in a statistically significant plasma iron increase after only two hours from oral administration of low dose of Ferrous elemental iron (60 mg). This fast plasma iron increase together with the low oral iron administration confirms the interesting profile of FERALGINE® in terms of efficacy and safety that could be useful for IDA patient’s management. Not only the differences between patients affected by intestinal mal absorption but also the differences in oral absorption of iron belonging to different salts could be pointed out by using OIAT. Andersen and Coll demonstrate different results between iron salts in homogenous group of patients [9]. Oral Iron Absorption Test could be a simple, non-expensive and quick procedure to check for the right oral iron preparation to be given to IDA patients to improve their domiciliary compliance to treatment. The remarkably results obtained with low dosage of FERALGINE® in this trial could ascribe to this new compound a key role for IDA patient’s therapy, especially when other oral iron salts have been interrupted because of gastrointestinal discomfort.
Several variants of OIAT in heterogeneous study populations have been published [28]. Iron absorption studies in iron deficient patients and healthy persons have been performed using iron radioisotopes, but they are no longer used in clinical practice due to radiation exposure and logistical difficulties [30,31]. There are reports of healthy volunteers receiving 50 mg of bivalent sodium ferrous citrate without a significant iron increase, whereas the administration of 100 - 150 mg leads to an increment of plasma iron [32]. The most commonly used OIAT in clinical practice in Iron Deficient patients is the administration of 200 mg bivalent iron in fasting condition, measuring the increase of plasma iron 2 and 4 hours later, although a validation of this test is lacking [28]. Additionally, there are differing statements about the normal increase after oral iron intake in this iron deficient population, for example a relative increase of 50% to 200% after 2 or 4 hours or an absolute increase of at least 9 µmol/l respectively [28]. Other Authors talk about an increase of 100 µmol/l after three hours as gold standard for optimal oral iron absorption [29-31]. Taking into account the 4 to 1 ratio between Ferrous Bysglicinate Chelate and other Ferrous salts [13-16] and the new “theoretical” quick and more extensive bioavailability of FERALGINE® if confronted with Ferrous Bysglicinate alone, we performed an OIAT in 14 consecutive adults IDA patients treated with “only” 60 mg of elemental iron belonging to FERALGINE® (instead of 200 mg recommended) and checking to plasma iron after “only two hours” (instead of the recommended three hours). Since the new compound containing Ferrous Bysglicinate chelate and Alginic Acid co-processed by spray-drying technology seems to be more available than Ferrous bysglicinate Chelate alone we postulated than only little quantity of elemental iron could be enough to demonstrate the same extent in plasma iron obtained following the most recommended international guidelines published on OIAT. Ferrous Bysglicinate Chelate alone has just proven to be effective in IDA children affected by Celiac Disease after three hours of the OIAT [15]. The results of our study confirmed the “preclinical data” on FERALGINE showing a statistically significant improvement in plasma iron values (increase of plasma iron concentration = 100 µmol/l, T Students Test for paired samples p<0.00001 between T0 and T2) only after two hours of the OIAT. Considering the two gold standard for OIAT (150 - 200 mg of oral administration of elemental iron and increase in plasma iron of 50% after two hours or 100 µmol/l after three hours) it seems that FERALGINE® could be faster absorpted and even more than other Ferrous Iron salts, included Ferrous Bisglycinate alone (Figures 4 and 5).
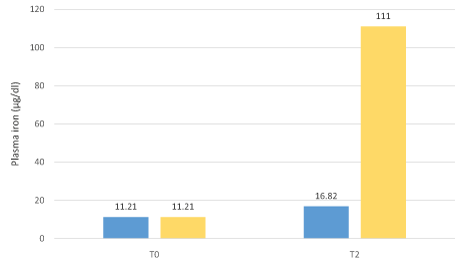
Figure 4: Expected plasma iron increase in IDA patients versus FERALGINE plasma iron increase.
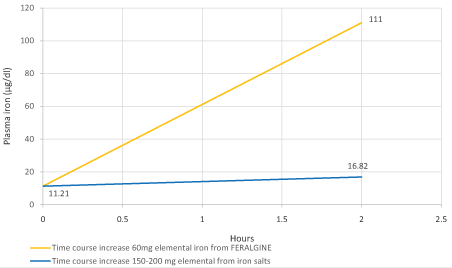
Figure 5: Expected theoretical plasma iron level after 2 hours from 200 mg of elemental iron oral salts administration (blue lines) versus experimental FERALGINE 60 mg elemental iron oral administration (yellow line).
Download Provisional PDF Here
Article Type: Research Article
Citation: Rondinelli MB, Di Bartolomei A, De Rosa A, Pierelli L (2017) Oral Iron Absorption Test (OIAT): a forgotten screening test for iron absorption from the gastrointestinal tract. A case series of 14 Iron Deficiency Anemia (IDA) patients treated with FERALGINE®. J Blood Disord Med 2(1): doi http:// dx.doi.org/10.16966/2471-5026.114
Copyright: © 2017 Rondinelli MB, et at. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access