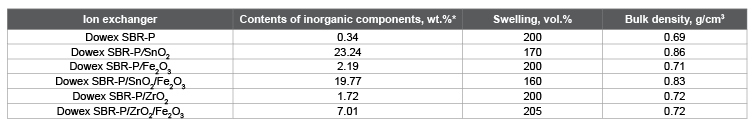
Table 1: Contents of inorganic components, swelling and bulk density of hybrid materials based on anion exchanger Dowex SBR-P Note: Contents of inorganic components introduced were determined by weight of the residue after burning at 700°C [7].

Kolomiyets YO* Belyakov VN Palchik AV Maltseva TV
Vernadsky Institute of General and Inorganic Chemistry, 32-34 Acad. Palladina Ave., 03680, Kyiv, Ukraine*Corresponding author: Kolomiyets YO, Vernadsky Institute of General and Inorganic Chemistry, 32-34 Acad. Palladina Ave., 03680, Kyiv, Ukraine, E-mail: kolomiyetsy@gmail.com
A hybrid organic-inorganic ion-exchanger which consists of the organic anion exchanger Dowex SBR-P and hydrated oxides of Sn (IV), Zr (IV) and Fe (III) was synthesized by sol-gel precipitation. The adsorption properties of hybrid ion-exchanger with various amount of inorganic component were studied towards the anions of arsenic (V). The content of arsenic in the solution and medium acidity were varied and their sufficient influence on the sorption capacity of ion-exchanger was found. In neutral and acidic media organic anion-exchanger Dowex SBR-P found to be ineffective. Introduction of the Sn (IV) and Sn (IV)/Fe (III) hydrated oxides effectively extends the range of action of the anionexchanger to acidic media and significantly improves the adsorption of arsenic (V) from the diluted solutions.
Hydrated Oxides; Organic anion-exchanger Dowex SBR-P; Iron oxides; Arsenic (V) Anions
Expanding the scope of the ion-exchange sorption technology to effectively extract expensive or toxic ionic components from aqueous solutions is largely determined by the presence of functional ion-exchange materials with the desired set of properties. One such property is the selectivity of the resin towards a target ion in salt solutions of complex composition.
A number of studies [1,2], carried out in recent years, has shown promising opportunity to improve the properties of ion exchange resins by introducing them to the sorption-active inorganic components. Presence of an inorganic ion exchanger inside of organic matrix allows to greatly improving the selective properties. It was shown that introduction of iron oxides in the ion exchange resin lead to creation a hybride material with a good selectivity towards anions of arsenic [3-5].
The purpose of this study was to estimate the effect of precipitation of hydrated oxides of tin (IV) and ferric (III) as inorganic sorption-active components in a matrix of a polymeric anion exchanger Dowex SBR-P on the physico-chemical and adsorptive properties towards ions of arsenic (V).
Strongly basic anion exchanger Dowex SBR-P was selected for studies, which is manufactured by Dow Chemical Company, with quaternary amines as functional groups and with the following characteristics: minimum exchange capacity –1,2 mol•eq•dm3 , water content –53- 60%, the average grain size –0,3 –1,2 mm, grain density –1,08 g•cm3 , bulk density –0,69 g•cm3 , the working pH range -0-14, the working temperature range up to 100°C [6].
For introducing of hydroxides of Sn (IV), Fe (III), Zr (IV) or the mixture of hydroxides into the organic polymer matrix the ion-exchange resin Dowex SBR-P was impregnated with an aqueous solution or a mixture of solution of metal chlorides. A weighed portion of 10 g of ion exchange resin Dowex SBR-P were placed in 50 mm3 of a 1 M aqueous solution of metal salts and heated with stirring during 1 hour, and then the beads was filtered, washed with distilled water, placed into 30 mm3 of 25% NH4 OH solution and stirred during 24 hours. Upon completion of these operations, the granules of ion exchange material were filtered off, thoroughly washed with distilled water and air dried to constant weight. Some properties of the anion-exchanger Dowex SBR-P and hybrid ionexchanger are shown in table 1.

Table 1: Contents of inorganic components, swelling and bulk density of hybrid materials based on anion exchanger Dowex SBR-P Note: Contents of inorganic components introduced were determined by weight of the residue after burning at 700°C [7].
Solutions for sorption studies were prepared by dissolving one-aqueous sodium dihydrogen arsenate (NaH2 AsO4 • H2 O) of high purity category in distilled water. The content of arsenic ions in the solution was determined by atomic absorption spectrometry flame device Pye Unicam SP9.
Sorption study performed under static conditions at 20°C. For this purpose, a samples of 0.2 g of the sorbent were placed into a flasks in which was added 100 mm3 of an aqueous solution of a predetermined concentration of pentavalent arsenic and then were placed in an thermostat for shaking during 8 hours. In preliminary experiments it was established that such time is sufficient to reach of adsorption equilibrium. To investigate the influence of pH on the absorption of ions of arsenic (V) equilibrium pH was adjusted to predetermined values by acidifying 0.1 M solution of nitric acid.
Results of an estimating of the influence of the introduction of the inorganic oxide component to the organic anion absorbency Dowex SBR-P towards the anions of arsenic (V) from solution with concentration 500 mg•dm3 in a wide range of pH are shown in Figure 1. It can be seen that the introduction of relatively small amounts (up to 23% by weight) of inorganic oxide components SnO2 and Fe2 О3 :SnO2 increases the magnitude of the sorption capacity at 50% in slightly acidic solutions (pH 4-6) and to a much lesser extent - 5-10% - in the alkali ones (pH 10-11). It should be noted that the absorption capacity of hybrid ion exchangers does not affect the acidity of arsenic salt solution (pH= 5-11), while the initial organic resin demonstrated a gradual increase of the anion adsorption capacity with increasing pH.
Data obtained in a more detailed study of the sorption properties of hybrid ion-exchangers are shown in Figure 2 in the form of sorption isotherms of ions As (V) at different equilibrium pH of solutions.
A detailed analysis of the data shown in Figures 2-4, makes it possible to identify some of the most characteristic results about influence of the oxide component introduction in the anion-exchange matrix Dowex SBR-P on the adsorption properties of hybrid sorbents towards to ions of As (V).
In the case of hybrid ion exchangers maximum adsorption value can be achieved at considerably smaller content of As (V) ions in the equilibrium solution, as clearly demonstrates the adsorption isotherms for acidified solutions. We can assume that since the introduction of inorganic oxide increases the slope of sorption isotherms, the saturation of sorption material occurs at much lower concentrations due to more complete extraction of As (V) ions.
Influence of the difference in the chemical composition of oxides introduced on absorbency of hybrids is observed only for adsorption in alkaline solutions. The curves in Figure 2 show that in solutions with a low content of As (V) exchange resin with the together introduced hydrated oxides Fe2 O3 and SnO2 demonstrates maximal adsorption capacity in comparison with other adsorbents. However, differences in the sorption behavior of the initial organic anion exchanger and the anion exchanger containing only SnO2 , disappears at the increase in the acidity of solutions (isotherms became almost identical). It can be seen that significant differences in the sorption behavior of the original anion resin and hybrid anion exchangers are observed at the acidification of the equilibrium solutions.
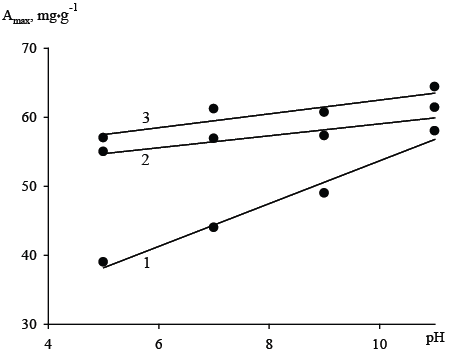
Figure1: Influence of acidity of the solution (500 mg • dm-3 ) on the sorption capacity (Amax) of the original Dowex SBR-P (1) and hybrid ion exchangers containing hydrated tin oxides Dowex SBR-P/SnO2 (2) and tin and iron oxides Dowex SBR-P/SnO2 /Fe2 O3 (3).
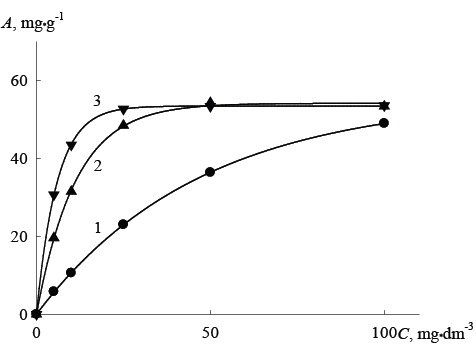
Figure 2: The isotherms of As (V) ion adsorption by hybrid exchangers and initial organic resin from solutions at pH 9: 1–Dowex SBR-P, 2-Dowex SBR-P/SnO2 , 3 - Dowex SBR-P/SnO2 /Fe2 O3 , A-absorption, C-equilibrium concentration of As (V) ions in the solution.
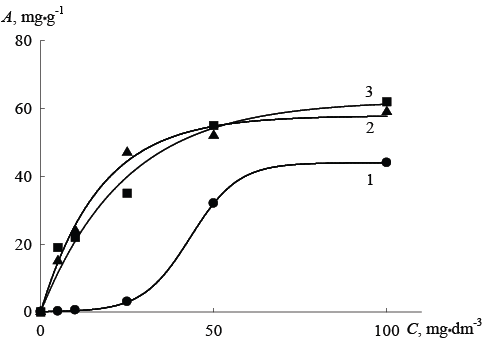
Figure 3: The isotherms of As (V) ion adsorption by hybrid exchangers and initial organic resin from solutions at pH 7: 1-Dowex SBR-P, 2-Dowex SBR-P/SnO2 , 3-Dowex SBR-P/SnO2 /Fe2 O3 , A-absorption, mg•g-1; C-equilibrium concentration of As (V) ions in the solution.
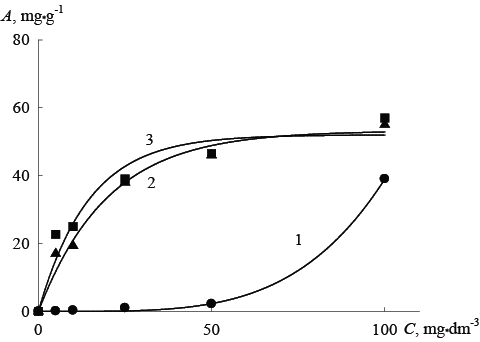
Figure 4: The isotherms of As (V) ion adsorption by hybrid exchangers and initial organic resin from solutions at pH 5: 1-Dowex SBR-P, 2-Dowex SBR-P/SnO2 , 3-Dowex SBR-P/SnO2 /Fe2 O3 , A-absorption, mg•g-1; C-equilibrium concentration of As (V) ions in the solution.
In dilute solutions adsorption isotherms for anion exchange resin Dowex SBR-P clearly demonstrate a significant decrease of the absorption value with decreasing pH equilibrium solution, while for the hybrid ionexchange resins such behavior is not typical.
Figure 5 shows the dependence of the ion distribution coefficient of As (V) ions on the equilibrium pH of the solution. High values of the distribution coefficients, reaching ~5000 for hybrid exchangers based on anion resin Dowex SBR-P, and significantly higher than the values for the initial organic anion exchanger, lead to the assumption that such materials may be effective for the treatment of aqueous solutions which contents an anions of As (V). No influence of pH on the distribution coefficient of As (V) ions observed for hybrid sorbents indicates its high selectivity in spite of the increase of content in solution competing anion OH- .
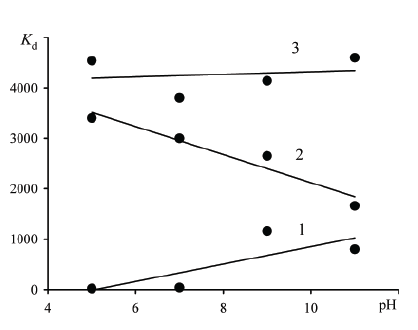
Figure 5: The distribution coefficient of As (V) ions versus the equilibrium pH calculated for concentration of solution 5 mg•dm-3: 1-Dowex SBR-P, 2-Dowex SBR-P/SnO2 , 3-Dowex SBR-P/SnO2 /Fe2 O3
Figure 6 shows the dependence of the sorption of As (V) ions on the pH in solution with a concentration 100 mg• dm-3 NaH2 AsO4 • H2 O and 0.1 mol • dm-3 KCl. Organic anion exchanger under these conditions is much less selective towards arsenic (adsorption value is less than 1 mg • g-1). Organic-inorganic hybrid exchanger with iron and zirconium oxides individually introduced show medium absorption selectivity towards arsenates. However, the sorbent with iron oxide introduced is ineffective in a neutral medium while sorbent introduced zirconia exhibits maximum selectivity to arsenate in a neutral environment. High selective adsorption values (the value of sorption reaches ~14 mg • g-1) for these conditions were obtained for hybrid materials based on the anion exchanger Dowex SBR-P and at the same time introduced the iron oxides and zirconium.
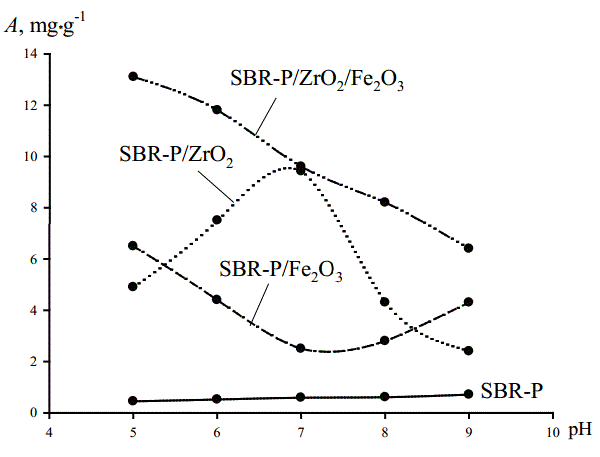
Figure 6: Effect of acidity of the solution (100 mg•dm-3 NaH2 AsO4 • H2 O and 7,5 g•dm-3 KCl) on the As (V) ions adsorption on the Dowex SBR-P and hybride ion exchangers containing hydrated oxides of zirconium Dowex SBR-P/ZrО2 , hydrated oxides of iron Dowex SBR-P/ Fe2 О3 , hydrated oxides of iron and zirconium Dowex SBR-P/ZrО2 /Fe2 О3
From hybrid adsorbents under investigation the Dowex SBR-P/ SnО2 was chosen for study of influence of pH value on adsorption of arsenate ions, and Dowex SBR-P/ZrО2 was chosen for study of influence of competition of KCl on adsorption of arsenate ions. In both cases competitive ions (NO3 - , Cl- ) influence strongly on adsorption of arsenate ions by surface of organic resin Dowex SBR-P and influence more weakly on adsorption of one’s by hybrid adsorbents. It was interesting to study influence of high concentration of salt (KCl) in case Dowex SBR-P/ZrО2 , because the quantity of inorganic component added in this case was very small (Figure 7). Obviously in case of SnO2 addition the selectivity should be best due to sufficient amount of inorganic component. The result obtained show in case of ZrO2 addition the selectivity of hybrid adsorbents is good enough too.
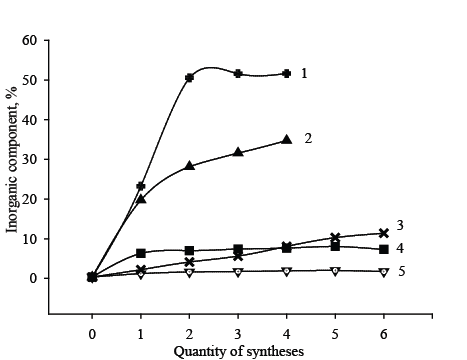
Figure 7: Dependencies of inorganic component content on the quantity of syntheses: 1-SBR-P/SnО2 , 2-Dowex SBR-P/SnО2 / Fe2 О3 , 3-Dowex SBR-P/ Fe2 О3 , 4-Dowex SBR-P/ZrО2 / Fe2 О3 , 5-SBR-P/ZrО2
So after precipitation of hydrated oxides inside the granules of organic resin the reactivity of surface towards ions of arsenate increases.
Thermogravimetry studies shown that at 150°C the weight loss is 65% for organic resin Dowex SBR-P and about 15% for all hybrids under investigation. It can be suggested from the results that water inside organic resin after formation of hybrid adsorbents changes its state, or, more accurately, it changes degree of its chemical adsorption and ionization. This process is caused by surface OH- groups inside the pores of oxides, which can react with water with the formation of a positively or negatively charged surface. That is why selectivity of adsorption towards multiply charged ions is better for hybrid adsorbents in comparison to organic resin. In addition, ions of As (V) contents an O-atoms, which can react with surface groups of oxides or with ionized water. So after precipitation of hydrated oxides inside the granules of organic resin the reactivity of surface towards ions of arsenate increases due to charged selective pores formation.
Thus, this study showed the possibility of a significant improvement in the sorption properties of the anion exchanger Dowex SBR-P towards to the ions As (V) by introducing into the polymer matrix of inorganic sorption-active components-hydrated oxide of tin, iron, and double oxides tin and iron, double oxides zirconium and iron. The influence of inorganic components to improve the selectivity of absorption in dilute solutions was found, while reducing the impact of the value of the acidity of solutions on sorption capacity is observed.
Download Provisional PDF Here
Article Type: Research Article
Citation: Kolomiyets YO, Belyakov VN, Palchik AV, Maltseva TV (2016) Effect of Incorporation of Hydrated Oxides of Sn (IV), Zr (IV) and Fe (III) in a Matrix of the Anion Exchanger Dowex SBR-P on the Sorption Capacity towards the Arsenic (V) Anions. Int J Water Wastewater Treat 2(2): doi http://dx.doi. org/10.16966/2381-5299.120
Copyright: © 2016 Kolomiyets YO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access