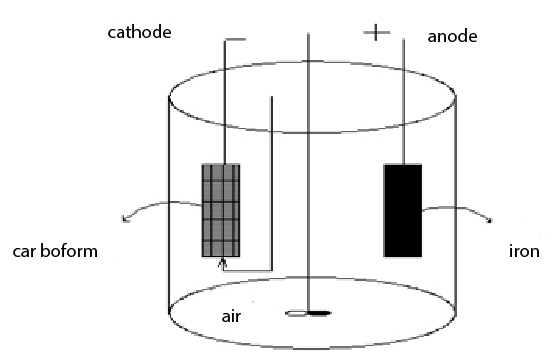
Figure 1: Schematic diagram of the bipolar electro-Fenton system

Jin Zhang* Mihua Shao Ping Tao
School of Environmental Science and Engineering, Dalian Maritime University, Dalian, PR China*Corresponding author: Jin Zhang, School of Environmental Science and Engineering, Dalian Maritime University, Dalian, PR China, E-mail: zhangjin7986@163.com
This study aimed to evaluate the treatment for Congo Red wastewater with Fe-ACF bipolar electro-Fenton technology. By controlling the influence factors, the treatment efficiency for Cong Red with the Fe-ACF bipolar electro-Fenton process was studied. The results showed that the electrolysis voltage, electrolyte concentration, pH value and electrolytic time have influence on the decolorization of Congo Red wastewater. In the experimental range, the optimum pH was 7. Under the optimum pH value, and at voltage of 25V, electrolyte concentration of 10 g/L, the decolorization ratio for Congo Red wastewater could be up to 100%, and COD removal rate could reach above 95%. Under differ pH value, Congo Red exit at different forms, which affect the oxidation extent for Congo Red. The bipolar electro-Fenton is a valid technology for treatment for dye wastewater.
Fe-ACF bipolar electro-Fenton; Congo red; Dye wastewater; Advanced oxidation
With the development of textile and dye industry, the emission of dye wastewater increases continually [1,2]. Textile industry effluents are one kind of the most critical environmental problems in the developing countries [3]. Azo dyes are extensively utilized in textile industry owing to the ability of their reactive groups to bind textile fibers by covalent bond formation [4]. Most of the azo dyes are characterized as toxic to aquatic life and mutative and carcinogenic to human, and may cause longterm environmental and ecological impacts without proper treatment [5]. Among them, Congo Red (CR) has been extensively used as a synthetic organic dye in textile industry. The degraded by-products of CR containing toxic aromatic amine compounds, which are carcinogenic and found to be harmful for skin, eye, blood and reproductive cell of human body [6]. This kind of wastewater has quality of high salinity, strong color, high chemical oxygen demand (COD) and highly fluctuating pH etc [7,8]. Dye wastewater has been seen as one of the intractable industrial wastewater. Azo dyes are resistant to biodegradation in conventional aerobic treatment processes, and in recent years, with the development of dye technology and upgrading of dyeing products, the treatment for dye wastewater become more and more difficult. The traditional process for wastewater treatment has been unable to meet the requirements for dye wastewater. Adopting some new process and improve the treatment efficiency for dye wastewater is the most urgent problem.
In recent years, advanced oxidation technologies have been described as efficient procedures for obtaining high oxidation yields from several kinds of organic compounds [9-11]. As one of advanced oxidation technology, electro-Fenton technology has been researched for treatment for refractory organic wastewater, and has achieved preferable development [12-15]. Electro-Fenton is mainly relied on an in situ and catalytic production of Fenton’s reagent – a mixture of hydrogen peroxide (H2 O2 ) and ferrous iron (Fe2+) to produce hydroxyl radicals to react with organic pollutants in water and finally leading to their destruction [16,17]. In electro-Fenton, Fe2+ and H2 O2 are generated in situ, and H2 O2 is electrogenerated in the presence of catalytic amounts of iron ions, thus leading to
the formation of the strong oxidant HO▪ [18-20].
O2 + H+→ H2 O2 …… (1)
Fe2+ + H2 O2 → Fe3+ + HO▪ + OH−
…… (2)
HO▪ + H2 O2 → H2 O+ HO2 ▪ ……. (3)
Fe3+ + HO2 ▪ → Fe2+ + H+ + O2 ……. (4)
Fe2+ + HO2 ▪ → Fe3+ + HO2 - …… (5)
Fe2+ + ▪OH → Fe3+ + OH- ……. (6)
Serkan Sahinkaya [21] studied the combination of electro-Fenton oxidation process with sonication for the degradation of C.I.Reactive Black 5 and removal of chemical oxygen demand (COD) from synthetic textile and shown that at optimum pH of 3, DC current of 0.25 A, H2 O2 dosage of 800 mg/L, high RB 5 degradation and COD removal could reach. Ricardo Salazar et al. [22] studied the degradation of disperse azo dyes from waters by solar photo electro-Fenton and shown that electroFenton process along with the additional photodecomposition of Fe(III) complexes with by-products allowed the almost total mineralization of both dye solutions. Ali Ozcan et al. [23] has use carbon sponge as cathode material for the electro-Fenton process to degradation of synthetic dye basic blue 3 and received 91.6% of TOC removal.
In this paper, a bipolar electro-Fenton process was put forward to treat Congo Red wastewater. Through systematic analysis, the feasibility of the technology has been discussed.
Fe-ACF electro-Fenton process was performed in batch mode in an undivided glass cell under vigorous stirring performed by a magnetic stirrer with 1000 mL of solution. The anode was a sheet iron of size of 10 cm*8cm*0.5cm, and cathode was an activated carbon fiber of size of 10cm*8cm. Compressed air was fed to the cathode by a air pump (Figure 1). Electrolyses were performed with a DC power supply. The Congo Red wastewater was simulated water with Congo Red dissolved in water, and 0.1M Na2 SO4 solution was used as supporting electrolyte.
Electrolyses were performed at room temperature, and pH of water was adjusted by addition of solution of 0.1 mol/L sulfuric acid and sodium hydroxide. The Congo Red concentration was measured with spectrophotometry.
In this study, the effects of pH, concentration of supporting electrolyte, electrolysis time and operational voltage were investigated. The results of this study are given bellow. The initial concentration of Congo Red was 0.7 mmol/L.
PH has some effect on treatment efficiency of electro-Fenton process. In general, for high production of .OH, the pH of water is controlled at about 2-3. In this group of experiments, the concentration of electrolyte was constant at 10 g/L, operational voltage was 25V and the pH was changed to 2.0, 5.0, and 7.0. Note that experiments were repeated at least 3 times at each pH value exactly in identical conditions and the average values were used in calculations. The effect of pH on removal efficiency of Congo Red was shown in Figure 2.
It is obvious from average Congo Red removal at different pHs that the pH has effect on Congo Red removal efficiency by process of the bipolar electro-Fenton. The removal efficiency of Congo Red at pH 7.0 was high in the whole electrolysis process, while was lower at first at lower pH of 5.0 and 2.0, but then increased with the process of electrolysis.
This may be because that when the pH of water was at 7.0, Congo Red exists in form of sulfonic acid sodium salt (Figure 3), which is easy to be oxidized. While with the pH decreased, the Congo Red forms changed. Especially when pH was lower than 3.0, the Congo Red exits in form of in-adjacent quinoid salt (Figure 4) with –NH2+, which is resistant to oxidation. Thus, the removal efficiency of Congo Red at lower pH was relative low at first. But with the process of electrolysis, the oxidation factor (.OH) increased, the removal efficiency of Congo Red increased.
In traditional, the advanced oxidation process was conducted in acidic pH conditions (pH value of 2.0-5.0) for high yield of the free hydroxyl radical, and which caused great limitations for the application of the technology.

Figure 1: Schematic diagram of the bipolar electro-Fenton system
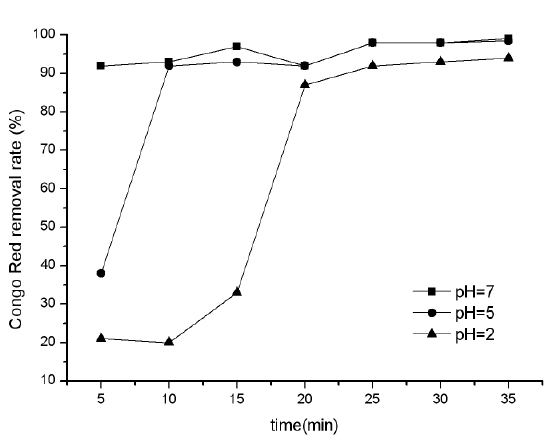
Figure 2: Removal ratio for Congo Red at different pH
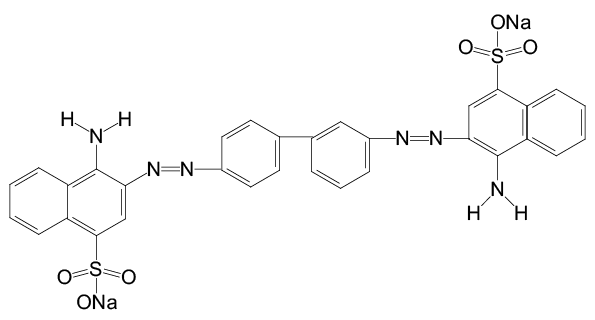
Figure 3: Chemical structure of Congo Red at neutral pH
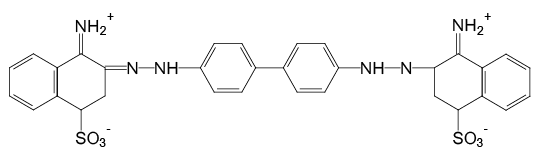
Figure 4: Chemical structure of Congo Red at acidic pH (smaller than 3)
In this experiment, the optimum pH value was 7.0, which break through the limitation of acidic pH, and which is very advantageous for the development of the technology.
As a result of the experiments discussed above, pH was kept constant at 7.0, operational voltage was 10V, and the concentration of supporting electrolyte was conducted at 5, 10, 15, 20, 25 and 30 g/L to invest the effect of electrolyte concentration. Figure 5 shows the removal efficiency for Congo Red at different electrolyte concentration, and Figure 6 shows the removal ratio for COD. It could be seen from the two figures that the removal efficiency of Congo Red increased with the increase of electrolyte concentration. The results of Figure 5 show that when electrolyte concentration was 5 g/L, the removal efficiency of Congo Red was very low at the whole electro process. But when the electrolyte concentration increased to 10 g/L, the removal efficiency of Congo Red increased obviously, and increased with the process of electro-Fenton.
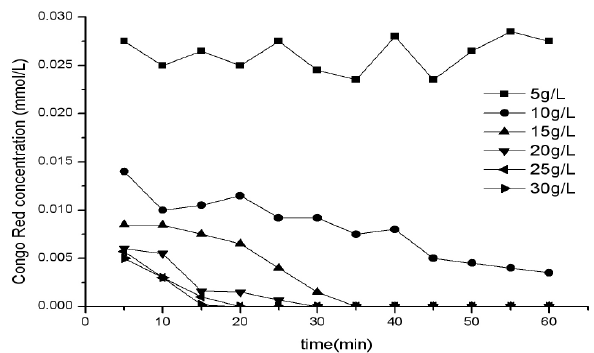
Figure 5: Effect of concentration of supporting electrolyte on Congo Red removal
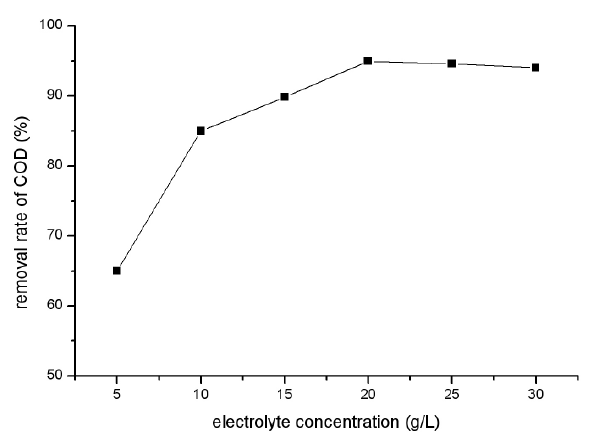
Figure 6: Effect of concentration of supporting electrolyte on COD removal
This may be because that the water was synthetic water from tap water, and the conductivity is very low. When the electrolyte concentration was low, the current efficiency is low, so the Fenton’s reaction is difficult to produce in the electrolysis process, so Congo Red can’t be oxidized and removed. While with the increase of electrolyte concentration, the current efficiency increased, and the Fenton’s reaction produced easily in the process of electro process, so Congo Red would be oxidized and removed. With the process of electrolysis, more and more .OH would be produced, and the removal efficiency for Congo Red increased.
In this group of experiments, the voltage changed (5, 10, 15, 20, 25 and 30V) and the pH was constant at 7.0, electrolyte concentration was kept at 10 g/L. The results of effects of voltage were shown in Figure 7. It is obvious that there is positive effect of voltage on the removal for Congo Red by process of the electro-Fenton. At low voltage of 5V, the removal rate of Congo Red was 74.3% at 5min, and decreased to 87.1% after 60min. With the voltage increased, the removal rate increased. At voltage of 10V, the rate increased from 80.3% at 5min to 97.1% at 60min. At 15V, the removal rate reached to 85.7% at 5min and then increased to 100% at 55 min. When at 20, 25 and 30V, the removal rate at 5min was 90%, 91.4%, 92.9% and increased to 100% at 45 min, 25 min and 20 min distinguish.
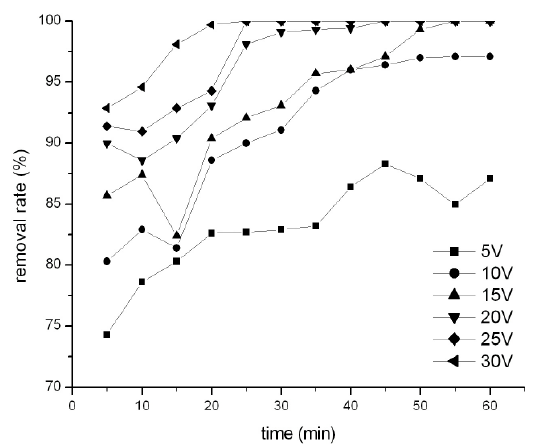
Figure 7: Effect of operational voltage
This work has shown that the Fe-ACF bipolar electro-Fenton process has good efficiency for Congo Red wastewater treatment. Many factors, such as pH, supporting electrolyte concentration, electrolysis time and operational voltage, affect the treatment efficiency for Congo Red wastewater. At experimental conditions, the optimum pH was 7.0. Operational voltage and supporting electrolyte condition had positive effect for treatment of Congo Red. The bipolar electro-Fenton process in this study is for instance capable of removing 100% of the color of Congo Red and up to 95% of COD. The technology of bipolar electro-Fenton process has break through the limitation of condition of acidic pH.
The authors thank to the supported of “the Dalian construction science and technology planning project of 2014” and “the Central gold project into the sea area, China, 2012 (Environmental)-Vegetation Repair and reconstruction of Shuangtaizi estuary wetland of Liaoning province”.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Zhang J, Shao M, Tao P (2015) Treatment for Congo Red Wastewater with Fe-ACF Bipolar ElectroFenton Technology. Int J Water and Wastewater Treatment 2(1): doi http://dx.doi.org/10.16966/2381- 5299.114
Copyright: © 2015 Zhang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access