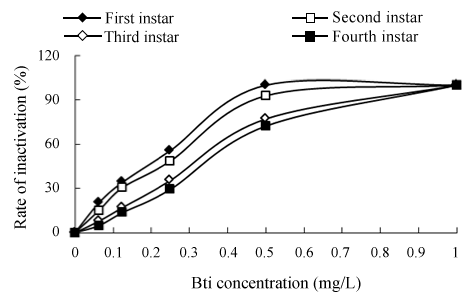
Figure 1:Inactivation effects of Bti on Chironomid larva at pH 7.0 and 25°C

Jing Pan1* Long Yu2 Qing-Chi Shan1 Jing-Xiao Yu1 Yi-Jing Guo1
1College of Chemical and Life Science, Shenyang Normal University, 253, Huanghe Street, Shenyang 110034, China*Corresponding author: Jing Pan, College of Chemical and Life Science, Shenyang Normal University, 253, Huanghe Street, Shenyang 110034, China. Tel: 8602486593335; Fax: 8602486592584; E-mail: crystalpan78@126.com
The excessive propagation of Chironomid larva in waterworks is a difficult problem. The inactivation of Bacillus thuringiensis subsp. israelensis (Bti) on different instar Chironomid larva was studied using distilled water as test sample. Furthermore, batch experiments were performed to analyze the influence of pH value, liquid chlorine, and water temperature and ammonia nitrogen on inactivation efficiency of Chironomid larva with Bti. Based on it, removal effects on Chironomid larva in the actual raw water and pre-chlorination coagulation sedimentation water were evaluated. The results showed that Bti possessed good inactivation performance to all larval stages of Chironomid. The 24 h semi-lethal concentration of Bti to the third instar Chironomid larva was 0.423 mg/L. The inactivation rate at pH 9.2 was significantly higher than that at pH 4.4 and 7.1. The liquid chlorine had negative effects on inactivation, indicated by the decreased inactivation rate from 74.22% at liquid chlorine concentration of 0 mg/L to 15.5% at 2.5 mg/L when Bti concentration was 0.5 mg/L. Water temperature ranging from 15℃ to 35℃ did not affect the inactivation efficiency of Bti. At Bti concentration of 0.125 mg/L, the inactivation rate significantly increased from 17.32% at ammonia nitrogen concentration of 0 mg/L to 53.26% at 6 mg/L. The inactivation rate of Chironomid larva was significantly higher in actual raw water than that in pre-chlorination coagulation sedimentation water. It could conclude static immersion with Bti solution in sedimentation tank is an effective method to control Chironomid larva.
Chironomid larva; Bacillus thuringiensis subsp. israelensis; Inactivation; Water
Chironomids are nematoceran Diptera closely related to mosquitoes, and are a diverse group that occupy a range of environmental niches and utilize a wide range of food sources. Chironomids are often used as indicators of water quality, but some species are pests. Chironomids have four life stages: egg, larva, pupa and adult. The larval stage consists of four instars (juvenile stages), and the larva shed their exoskeleton and increase in size to the next instar. The first instar larvae is plankton, while older instars (second, third and fourth larval stages) in most species migrate to the sediment and build tubes constructed from detritus, algae and sediment particles [1]. Chironomid larva can be found both in lentic and lotic environments, usually in organically enriched waters. Chironomid larval densities rapidly increased in water source such as reservoir or fresh lake due to eutrophication, which induced first instar larva in source water to enter drinking water treatment system. There are many serious accidents of Chironomid larva pollution in the water treatment system in the Britain, USA and China [2]. Although there are no indications that these organisms pose a threat to public health, their presence is still not appreciated because most people associate the organisms with low hygiene [3].
The first instar larva can easily penetrate sand filter and then goes into waterworks reservoir and municipal service pipe due to its motility [4,3]. Several researchers have investigated that Chironomid larva was difficult to inactivate using general drinking water utilities [4,5]. Chemical pesticides such as dichloro-diphenyl-trichloro-ethane (DDT), gammaxane, malathion and chlordane applied with the aim of eliminating mosquitoes have given rise to other serious problems [6]. Not only has mosquito resistance against these chemicals been reported, but the pesticides themselves present threats to both human health and the ecosystem. Unlike chemical insecticides, mosquitocidal bacilli used as larvicides are safe for animals and the environment. At present, Bacillus thuringiensis israelensis (Bti) and Bacillus sphaericus are being used in aquatic environments to control the populations of Aedes, Culex and Anopheles larva [6]. Bti, a ubiquitous spore-forming bacillus that produces a mosquitocidal crystal protein (6-endotoxin) consisting of five protoxins (cry4A, cry4B, crylOA, cryllA, and cytlA) has significant mosquito larvicidal activity against mosquitoes and blackflies [7]. The toxins act on susceptible larvae via the following cascade: (i) toxin ingestion by filter-feeding, (ii) toxin solubilization at alkaline pH in the midgut, (iii) proteolytic processing of active toxins and (iv) binding of the activated toxins to epithelial cells of the gut, leading to lysis of the cells and eventual larvae death. It is indicated that microbiological control is effective and economic on Chironomid pollution control in city water supply [8].
Limited information in the literature has shown that Bti can be very effective inactivation Chironomid larva in drinking water treatment. Experiments were conducted at bench-scale to evaluate the inactivation of Chironomid larva by Bti. Two specific objectives were discussed here as follows: one was to determine the usefulness of Bti on control of Chironomid larva in actual raw water and pre-chlorination coagulation sedimentation water of waterworks, the other was to examine influence of larval stage of Chironomid, pH value, liquid chlorine, water temperature and ammonia nitrogen on inactivation of Chironomid larva with Bti.
The stock solution of Bti was prepared by diluting commercial Bti powder (Hubei Kangxin Medicine Co., China) with distilled de-ionized (DDI) water. The stock Bti solution was usually diluted to obtain a concentration of about 250 mg/L in order to facilitate the addition of low concentration to the water samples.
Egg masses of Chironomid were initially obtained from a population collected in the Donghu reservoir (Shenzhen, China) and maintained in the laboratory for several generations. Chironomid larva was cultured in aerated 50 L stainless steel aquaria filled with aerated tap water. A 5 cm thick artificial sediment layer consisting of washed sand and cellulose was introduced at the bottom of the aquarium. Adult midges were confined using wooden cages covered with 1 mm mesh size metal net. Aquaria were placed under constant temperature (25°C) and photoperiod (15h light/9 h dark).
In order to investigate the effect of several important factors, such as larval stage of Chironomid, pH value, liquid chlorine, water temperature and ammonia nitrogen on the inactivation of Bti, the experiment was firstly undertaken in distilled water solution. Furthermore, the inactivation efficiency of Bti was studied in the actual raw water and prechlorination coagulation sedimentation water taken from the Shenzhen Water Treatment Plant (WTP). The characteristics of water are shown in Table 1.
One liter beaker was used as the test reactor. The third larval stage organisms (easy to pick, observe and don’t pupate) were transferred to each test jar with a glass pipette except in different larval stage of Chironomid experiment. Each beaker contained 100 larvas. Larva were regarded as dead if unable to make a sustained and coordinated response(at least two consecutive sinuate movements involving 25% or more of total body length) when grasped with a pair of fine forceps. Mortality data were pooled and corrected against the mean control mortality using Abbott’s formula. For each treatment group, five replicates were undertaken and no Bti was added to the control beaker. Ammonium chloride (NH4 Cl) and sodium hypochlorite (NaClO, 9% active chlorine) were used to make up ammonia nitrogen and liquid chlorine of distilled water solutions. NaOH or H2 SO4 were of reagent grade and used to adjust pH of distilled water solutions.
Various dosages of Bti were added into a given volume of distilled water solution and exposure time was 24h. Bti concentrations ranged from 0 to 1.0 mg/L. Experimental results for the inactivation of Chironomid larva with Bti at 25°C and pH 7.0 are presented in Figure 1. The data show that there was a significant difference in the inactivation rate between different larval stages of Chironomid. As a whole, the inactivation efficiency of Bti on the first larval stage was obviously better than that of the others. When 100% inactivation of the first instar larvae of Chironomid was obtained, the required Bti concentration was 0.5 mg/L, while 1.0 mg/L was required for due to high resistance of the fourth instar larvae of Chironomid. Zhang and Jin [9] reported that the inactivation rate of Bti on Chironomid larva decreased with the increasing of larval stage of Chironomid. The authors speculated that the decreased efficiency of Bti on bigger larval stage of Chironomid might be due to the incrassation of pellicle.
The third instar larva of Chironomid prone to pick and observe, which usually was made use of bioassays [10]. In this study, the 24 h semi-lethal concentration (LC50) of Bti to the third instar larva of Chironomid was 0.423 mg/L, which was the reference of Chironomid larva control in different water bodies.
Bench scale experiments were conducted to evaluate the impact of pH, in the range of 4.4-9.2, on the Bti inactivation. The inactivation effects at 25°C were observed and the results are shown in Figure 2. As depicted in Figure.2, the inactivation rate at pH 9.2 was significantly higher than that at pH 4.4 and 7.1 at all Bti concentrations. Weiser [11] examined the Bti inactivation of mosquito larva and presented evidence that alkalescence environment was in favor of enhancing the inactivation efficiency. Goldberg and Margalit [12] speculated that alkalescence solution increased the alkaline midgut milieu of mosquito larvae and the protein crystal (inactive protoxin) which was produced by the bacilli during sporulation was ingested by the target insect, and then proteases converted the protoxin into biologically active toxins in the alkaline midgut milieu. At last the toxins bound to cell-surface receptors (glycoprotein) of the midgut epithelial cells of mosquito larvae, which disturb the osmoregulatory mechanisms of the cell membrane, thereby swelling and bursting the midgut cells. Similarly, higher inactivation rate of Chironomid larva was presented under higher pH value in this study

Figure 1:Inactivation effects of Bti on Chironomid larva at pH 7.0 and 25°C
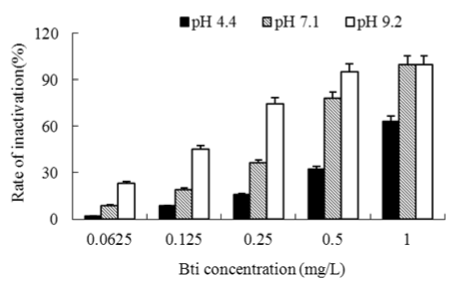
Figure 2: Effects of pH value on inactivation of Chironomid larva with Bti at 25°C
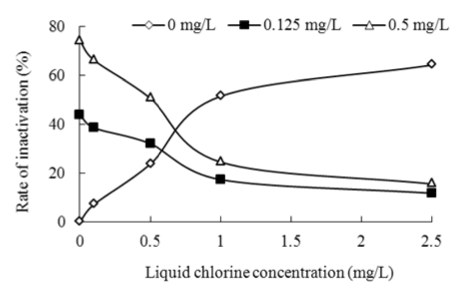
Figure 3: Effects of liquid chlorine on inactivation of Chironomid larva with Bti at 25°C, pH 7.0
Various dosages of Bti and liquid chlorine were added into a given volume of distilled water solution and exposure time was 24 h. Liquid chlorine from 0 to 2.5 mg/L. Experimental results for the inactivation of Chironomid larva with Bti at 25°C and pH 7.0 are presented in Figure 3. The data show that the inactivation efficiency of Bti was obviously better without liquid chlorine and liquid chlorine had negative effects on inactivation of Bti, indicated by the decreased inactivation rate from 74.22% at liquid chlorine concentration of 0 mg/L to 15.5% at 2.5 mg/L when Bti concentration was 0.5 mg/L. Liquid chlorine one of water treatment disinfectants has been reported to be effective in the inactivation of pathogenic organisms including Bti and Chironomid larva [13,5]. It could conclude that liquid chlorine counteracted the inactivation of Bti on Chironomid larva.
The concentrations of Bti were 0, 0.125 and 0.5 mg/L and exposure time was 24h. The inactivation of Chironomid larva over the water temperature range of 10–35°C was also conducted. Experimental results obtained for tests performed to assess the role of water temperature in the inactivation of Chironomid larva with Bti are shown in Figure 4. As depicted in the figure, the inactivation rate of Chironomid larva was 100% at 10°C with/ without Bti, because they couldn’t survive at this water temperature [14]. Water temperature had no influence on the inactivation of Chironomid larva with Bti from 15°C to 35°C. Osano et al. [15] reported that the inactivation of Cx. piliens with Bti was steady at the temperature ranged from 16°C to 33°C. The outbreak of Chironomid larva in waterworks occurs in summer. Therefore, control of Chironomid larva with Bti is feasible.
Various experiments were performed to investigate the inactivation effect of Bti at different ammonia nitrogen concentration. The concentrations of Bti were also 0, 0.125 and 0.5 mg/L and exposure time was 24h. It was found from Figure 5 that the ammonia nitrogen significantly affected inactivation of Chironomid larva with Bti and had inactivation effect on Chironomid larva. At Bti concentration of 0.125 mg/L, the inactivation rate significantly increased from 17.32% at ammonia nitrogen concentration of 0 mg/L to 53.26% at 6 mg/L. The suggested reason was that when the concentration of ammonia nitrogen raised, the concentration of ammonia increased simultaneously and ammonia had inactivation effect on aquatic organisms.
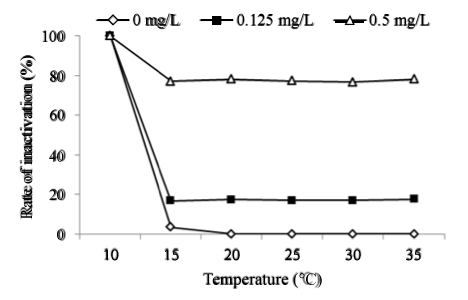
Figure 4: Effects of temperature on inactivation of Chironomid larva with Bti at pH 7.0
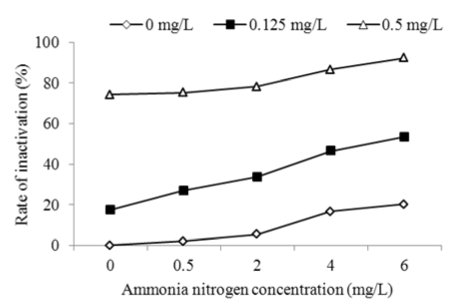
Figure 5: Effects of ammonia nitrogen on inactivation of Chironomid larva with Bti at pH 7.0
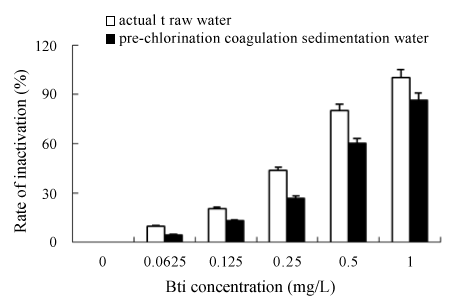
Figure 6: Inactivation of Chironomid larva with Bti in different water body
To better understand the behavior of inactivation process, actual raw water and pre-chlorination coagulation sedimentation water taken from the Shenzhen WTP were also used as the test samples. The comparative experiments were carried out at various concentrations of Bti, and the results are shown in Figure 6. The removal efficiencies of Chironomid larva were strengthened gradually with the increase of Bti concentrations regardless of the water type. But the inactivation rate of Chironomid larva was significantly higher in actual raw water than that in pre-chlorination coagulation sedimentation water. Sun et al. [16] reported when ammonia nitrogen concentration exceeded 1.0 mg/L, the death rate of Chironomid larva enhanced. In this experiment ammonia nitrogen concentration was 1.36 mg/L of actual raw water, which led to the inactivation efficiencies of Bti on Chironomid larva, was best of all experiment waters. In prechlorination coagulation sedimentation water there was residual chlorine (one of disinfectors), which had inactivity effect on Bti (above study) and Chironomid larva [13]. Many extraneous oxidant-demand substances exist in pre-chlorination coagulation sedimentation water including organic compounds, algae, bacteria, etc. Since most of them can be oxidized more easily than Chironomid larva by residual chlorine, the reaction between chlorine and Bti always proceeds prior to that with Chironomid larva. This result can be explained from the point of the body structure of Chironomid larva. Contrasted to common bacteria or virus, Chironomid larva have a special surface structure consisting of seven layers cell tissue, such as bottom membrane, epithelium, calcific layer, etc [5]. The body surface provides Chironomid larva stronger protection against chlorine. Chironomid larva cannot be effectively inactivated unless the oxidant destroys its surface structure by oxidation or directly penetrates through it into the body as to oxidize inner protein to lose the enzyme activity. So penetration of chlorine on surface structure of Chironomid larva is the key to thoroughly inactivate Chironomid larva [5]. At lower dose conditions, chlorine cannot effectively penetrate surface structure of Chironomid larva, but has inactivity effect on Bti, which led to the inactivation efficiencies of Bti on Chironomid larva was low in pre-chlorination coagulation sedimentation water. In view point of the inactivation of Bti on Chironomid larva of drinking water treatment, it is necessary not to use pre-chlorination for raw water, which on the other hand also decreases the efficiency of Bti. From the study, we could conclude that in the large-scale outbreak of chironomid larva, static immersion with Bti solution in sedimentation tank is an effective method to control them.
The extensive studies were conducted to evaluate the inactivation efficiency on Chironomid larva with Bti in bench-scale experiments. The results of the study demonstrated that Bti had good inactivation efficiency for all larval stages of Chironomid. The 24h semi-lethal concentration (LC50) of Bti to the third instar larvae of Chironomid was 0.423 mg/L. Alkaline environment is helpful to improve the inactivation efficiency of Bti to Chironomid larva. The higher liquid chlorine concentration was, the lower inactivation rate was achieved. The inactivation efficiency of Bti enhances with ammonia nitrogen. Water temperature ranging from 15°C to 35°C has no impact on the inactivation rate of Bti to Chironomid larva. The inactivation efficiency in actual raw water was higher by contrast with that in pre-chlorination coagulation sedimentation water with various Bti concentrations. In viewpoint of the inactivation of Bti on Chironomid larva of water treatment, it is necessary not to use pre-chlorination for raw water, which on the other hand also decreases the efficiency of Bti. Static immersion with Bti solution in sedimentation tank is an effective method to control Chironomid larva in summer.
This research is financially supported by the national natural science foundation of China (No.41001321)(No.41471394), Major Original Program in Shenyang Normal University (ZD201403), Ecology and Environment Research Center Director Foundation of Shenyang Normal University (EERC-T-201501). We thank the anonymous reviewers for their careful review and valuable suggestions on the manuscript.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Pan J, Yu L, Chi Shan Q, Yu JX, Guo YJ (2015) Inactivation of Chironomid larva in Water with Bacillus thuringiensis subsp. Israelensis. Int J Water Wastewater Treat 1(1): doi http://dx.doi. org/10.16966/2381-5299.103
Copyright:© 2015 Pan J, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access