Photo 1: Dumping place and reservoir in Damai site.


Nobuyuki Takahashi1* Toshiyuki Hibino1 Fumio Kiyono1 Hiroyasu Ichikawa1 Tuyen Trinh Van2 Tuan Minh Nguyen2
1Institute for Environmental Management Technology, National Institute of Advanced Industrial Science and Technology (AlST), 16-1 Onogawa, Tsukuba-shi 305-8569, Japan*Corresponding author: Nobuyuki Takahashi (2017) Institute for Environmental Management Technology, National Institute of Advanced Industrial Science and Technology (AlST), 16-1 Onogawa, Tsukuba-shi 305-8569, Japan, Tel: 81-29-861-8727; E-mail: takahashi-nobuyuki@aist.go.jp
In order to re-estimate and improve the decrease of organic compounds, the more detailed surveys in Damai landfill site located in a mountain area in Vietnam and some experiments using the leachate from this site were conducted. Afterward, a plan for improving the decrease of organic compounds in Damai site was proposed and O3/H2O2 was also conducted as an alternative method of fenton treatment. The obvious variations in water quality parameters were observed in all raw lechates. Regardless of the variations , BODs and COD were generally high in the raw leachate, showing the long-term outflow of organic compounds , and the ratio of BOD5 to COD were high of 0.17 ~0.32. The discharge criteria in Vietnam were met in the final discharged water. The extension of HRT from 20 to 40 h caused a higher extent of BOD5 removal, but no obvious effect on the removals of COD and color. The change of weight ratio of Fe to H2 O2 (O) from 2.3 to 3.4 caused a slight increase in the removal of BOD5 and COD, but no detectable difference in the removal of color. O3 /H2 O2 brought an obvious increase in the removal of color, and the adjustment of reaction time was an important factor. The treatment system containing O3 /H2 O2 will be an alternative method in Damai site.
Landfill leachate; Organic compounds; BOD5 ; COD; Color; Biological treatment; Fenton treatment; Coagulation; O3 /H2 O
According to recent economic development, the amount of waste has been rapidly increased in Vietnam. Tatsuo et al. [1] has reported that the amount of waste in Hanoi City will reach 2,415 t/day in 2002 and that in Ho Chi Minh City 4,210 t/day in 2000, respectively. The report published by the Ministry of Natural Resource and Environment (MONRE) in 2004 also simulates that the amount of solid waste will run up to 40 million t/year in 2015, 70 million t/year in 2020, and 90 million t/year in 2025, respectively [2]. This report also indicates that the total number of landfill site in Vietnam is 91, and that those equipped with a sanitary treatment system is only less than 20%. Moreover, the treatment systems in landfill sites have been indicated to be not necessarily operated efficiently [3].
In Vietnam, only slight amount of waste are treated through selection, separation by aeration, crash to pieces, and then recycled as fertilizer. However, almost all waste are collected together and directly dumped into landfill site without incineration from economical viewpoint [3]. As a result, the deterioration of water environment is seriously worried by the long-term outflow of biodegradable components from food residue. Tatsuo et al. [1] has also reported that municipal waste occupies approximately 70% of total amount of waste in Hanoi City and Ho Chi Minh City. In addition, due to natural conditions such as high moisture and much rainfall, a large amount of leachate may spill from the dumping area in landfill site. On the other hand , due to the enlargement of city area and the increase in awareness for the pleasant water environment , the new construction of landfill sites in the surrounding area of a city become more and more difficult recently. Therefore, recent landfill sites tend to be newly constructed in distant area from the center of a city and/ or mountain area.
Many surveys for the present state of landfill site in Vietnam have been conducted [3-5]. These surveys have showed that landfill leacheates in Vietnam generally contained highly concentrated organic compounds, and that the developments of more efficient treatments were needed. The shortage of fund and luck of appropriate knowledge for treatment technology have been also indicated [3]. However , these surveys are mainly targeted to landfill sites located in the suburbs of big cities, not to landfill sites in the mountain areas where possibly cause a serious effect on the water environment. Therefore, surveys targeted to landfill sites in the mountain areas are necessary from now on. From these backgrounds, Takahashi et al. [6] have conducted the survey of six landfill sites in Vietnam including Damai landfill site (Damai site) located in a northern mountain area in Thai Nguyen Province. These reports have also showed that fenton treatment was installed in some Damai site for the removal of non-biodegradable organic compounds and color. Fenton treatment has been applied to the treatment of landfill leachates as well as municipal wastewater [7-9], and some advantages such as no mass transfer limitation, no form of energy, and technological simplicity have been showed [8]. On the other hand, it has been generally indicated that there were some concerns in fenton treatment. That is, pH adjustment of around 3, later pH readjustment to neutral region for discharge, and treatment of Fe(OH)3 sludge produced during the reaction process are necessary. These procedures are heavy tasks in Damai site, because of shortage of both man power and area [3].
Both fenton treatment and ozonation with hydrogen peroxide (O3 /H2 O2 ) have been known as typical advanced oxygen processes (AOP) , and have a common characteristic of the production and use of hydroxyl radical (HO•) with high oxidation potential. Taking into account that H2 O2 has been already used in fenton treatment in Damai site, O3 /H2 O2 is an acceptable method in Damai site and will be capable of replacing fenton treatment. Nevertheless, few papers concerning to the treatment of landfill leachate by O3 /H2 O2 have been reported in Vietnam [10,11].
In this study, the more detailed surveys based on the previous report [6] were further conducted to estimate the present extent of the decrease of organic compounds in Damai site. In parallel of these surveys, some experiments were also conducted in Institute of Environmental Technology, Vietnamese Academy of Science and Technology (lET) for re-estimating the present extent of the decrease of organic compounds in Damai site, and afterward, a plan for improving the decrease of organic compounds was proposed and O3 /H2 O2 was also conducted as an alternative method of fenton treatment.
Damai site, which occupies 270,000 m2 area, is located in a northern mountain area approximate 100 km away from the center of Hanoi City, and has been operated by Urban Management Thai Nguyen Company since 2002. The domestic waste collected from Thai Nguyen City and its suburb was directly dumped into the site without incineration. Photo 1 shows the dumping place in Damai site. The dumping place is located in the upper area (left side in Photo 1), and the reservoir receiving the raw leachate in the down area (right side in Photo 1).

Photo 1: Dumping place and reservoir in Damai site.
Photo 2 shows the appearance of the treatment plant in Damai site. The green house in the right side is the administration center. Table 1 shows the capacity and the treatment flow of this treatment system. The raw leachate is temporarily stocked at the first reservoir, and if necessarily, the pH value is adjusted to neutral region. Afterward, the leachate is biologically treated for the removal of organic compounds, and fenton treatment and the coagulation/sedimentation using poly aluminum chloride (PAC) (fenton/PAC treatment) are further conducted for the removal of non-biodegradable organic compounds and color to meet the discharge criteria in Vietnam (B class of QCVN40) [6]. The final treated water is discharged to the nearby water body. It is easily expected that water quality parameters will be affected by rainfall in the site, because of a completely open type system.

Photo 2: Appearance of treatment plant.
Capacity (m3/d) |
Treatment flow |
400 |
Raw leachate→Reservoir→PH adjustment→ Aeration→ Sedimentation →Fenton→PAC→Coagulation/Sedilnentation |
Table 1: Present treatment system in Damai site
The present reaction conditions of biological treatment are 2,000~3,000 mg/L of mixed liquor suspended solid (MLSS) and 20 h of hydraulic retention time (HRT), respectively. The present reaction conditions of fenton/PAC treatment are as follows : the amount of FeSO4 •7H2 O added of 400 mg/L, that of H2 O2 (50%) added of 150 mg/L, reaction time of 30 min, and the amount of PAC added of 400~600 mg/L. The weight ratio of Fe to H2 O2 (O) is calculated as around 2.3.
The raw leachate was taken from the first reservoir. In the more detailed surveys, the each treated water were also taken after biological treatment, fenton treatment and the coagulation/sedimentation using PAC, and final reservoir , respectively . All sampling water were stored in a refrigerator in lET and kept on 4oC just before the analysis of water quality parameters.
All chemicals used were produced by Wako Pure Chemical industries (Japan). The mixed liquor was obtained from the aeration tank in Damai site. All experiments were conducted at an ambient temperature.
The raw leachate was first coagulated. Coagulation was conducted using a jar tester (JLT 6, Italy). Basic reaction conditions were referred to those of Damai site. 500 mg/L of PAC solution was used as a coagulant, and 5 mg/L of A 101 solution was also added as a coagulation aid. These coagulants were added to the raw leachate of 500 mL adjusted to pH value of 7 to 8. Rapid agitation at 150 rpm for 3 min and following slow agitation at 50 rpm for 3 min were conducted, respectively, and then, the solution was settled for 60 min.
The supernatant after coagulation was then biologically treated. The pH values of the solutions were controlled between 7 and 8 by adding 4 M-H2 SO4 or 2.5 M-NaOH solution. Figure 1 shows a schematic diagram of the biological treatment apparatus. Basic reaction conditions were referred to those of Damai site. The concentration of MLSS was definitely adjusted to 2,500 mg/L. Because there is a room for utilizing an unused aeration tank in Damai site, HRT in the aeration tank can be extended from 20 to 40 h. Therefore, HRT was adjusted to 40 h as well as 20 h.
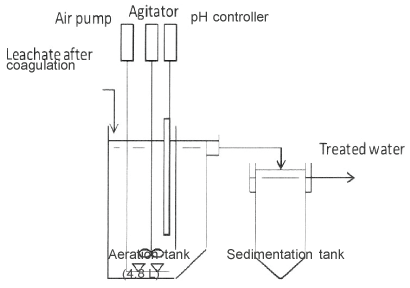
Figure 1: Schematic diagram of the biological treatment apparatus.
The biologically treated water was then treated by fenton treatment or O3 /H2 O2 . Basic reaction conditions in fenton treatment were referred to those of Damai site and the references [7-9]. The pH value of biologically treated water was adjusted to 3.5 using 4 M-H2 SO4 before fenton treatment. Afterward, 400 mL of FeSO4 • 7H2 O (concentration of 400 mg/L) and 100 or 150 mL of H2 O2 (30%, concentration of 100 mg/L) were added to the sample solution of 1,000 mL. The suitable weight ratio of Fe to H2 02 (O) in fenton treatment has been reported as around 3.5 [12], and in the case of 400 mg/L of FeS04 •7H2O and 100 mg/L of H2 O2 (30%), the weight ratio was calculated to 3.4. The solution was then mixed at 200 rpm for 20 min and settled for 30 min. The solution after the decantation of supernatant was taken as the treated water after fenton treatment.
O3 /H2 O2 , Which is an alternative method of fenton treatment, was also conducted using a semi-batch type ozonation apparatus illustrated in Figure 2. The biologically treated water of 1.5 L was placed in a reactor of 100 em height and 5.4 em diameter (total volume of 2.3 L) and added with 1.6 mL of H2 O2 (30 %). The sample solution was ozonated by bubbling oxygen gas containing 10 mg ozone/Lata flow rate of 4 Ll/min for up to 100 min. The molar ratio of H2 O2 to O3 after 100 min was adjusted to around 0.4 (0.6 after 60 min), because a suitable value has been reported to be in the range of0.5- 1.0 [13].
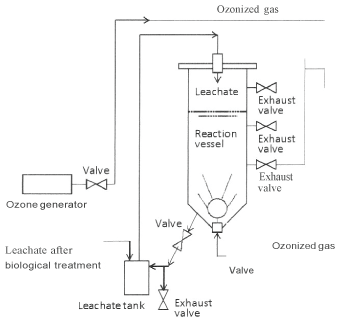
Figure 2: Schematic diagram of the ozonation apparatus.
Some laboratory-scale experiments of 1-F, 1-0, 2-F, and 2-0 were conducted in lET by combining these reaction conditions. Table 2 shows the reaction conditions in the new treatment system. The experiments are as follows;
1-F and 2-F: Coagulation using PAC→Biological treatment→Fenton treatment,
1-0 and 2-0: Coagulation using PAC→Biological treatment→O3 H2 O2 .
The reaction conditions of 1-F and 1-0 generally utilized those in Damai site and the objective is mainly the re-estimation of the present state in Damai. On the other hand, the reaction conditions of 2-F and 2-0 modified those in Damai site and the objective is the improvement of the decrease of organic compounds.
Fenton treatment and coagulation with PAC were separated in this new treatment system. Coagulation using PAC was placed as a pre-treatment for biological treatment. AOP is generally desirable to be placed in the late stage of treatment system, because HO• can react with many kinds of organic compounds. As a result, fenton treatment and O3 /H2 O2 was placed after biological treatment. In the experiment of 1-F and 1-0, the weight ratio of Fe to H2 O2 (O) and the reaction time in O3 /H2 O2 were adjusted to 2.3 and 100 min, respectively. In the experiment of 2-F and 2-0, the weight ratio of Fe to H2 O2 (O) was adjusted to 3.4, and the reaction time of O3 / H2 O2 was shortened from 100 to 60 min, because long-term ozonation was not so efficient [11]. Figure 3 shows the new treatment system and the sampling points.

Figure 3: New treatment system conducted in IET and sampling points.
5-Day biochemical oxygen demand (BOD5 ) , chemical oxygen demand (COD) , and color were measured according to the standard methods [14].
The concentration of heavy metals of as, total Hg, Cd, and Pb in May 2013 have been already shown to be less than those of the discharge criteria in Vietnam [6]. Afterward, the sampling items were targeted to BOD5 , COD, and color to investigate the decrease of organic compounds.
The more detailed surveys based on the previous report [6] and the analysis of the data was conducted to estimate the present extent of the decrease of organic compounds in Damai site. Figure 4 shows the variations of BOD s, COD, and color in raw leachates. A heavy rain fall is actually confirmed before the sampling in Dec. 2014, and this will be one of the main reasons for low values of water quality parameters.
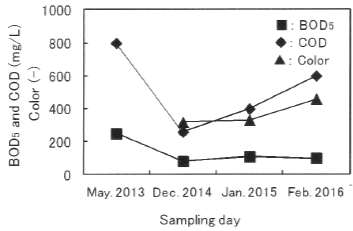
Figure 4: Variations of BOD5 , COD, and color in raw leachates.
As shown in Figure 4, the obvious variations in water quality parameters were observed at each sampling day. Regardless of the variations, BOD5 and COD representing the content of organic compounds were generally high in the raw leachates, showing the long-term outflow of organic compounds. The ratio of BOD5 to COD (BOD5 /COD) representing the biodegradability [7] were high of 0.17~0 .32. In addition, color were 320 in 2014 , 320 in 2015, and 460 in 2016 , respectively, and the appearance of all raw leachates was dark brown possibly derived from fumic substance. The concentrations of chlorine ion were very high between 320 and 618 mg/L.
Figure 4 indicates that further surveys will be necessary to estimate the present extent of the decrease of organic compounds by the each process, because the variations in raw leachate has observed. Only the raw leachate (①) was taken in the survey in 2013, and at the following survey, other three sampling water were also taken after biological treatment (aeration→sedimentation) (②), fenton/PAC treatment (fenton→PAC → coagulation/sedimentation) (③) , and final discharged water (④) , respectively.
Figure 5 shows the variation of water quality parameters in raw leachate and treated water after each process in Dec. 2014. As the reaction proceeds, BOD5 , COD, and color were gradually decreased. As a result, the discharge criteria in Vietnam of BOD5 50, COD 150, and color 150 were met in the final discharged water. The same trends were also observed in Jan. 2015 and Feb. 2016. Photo 3 shows the appearance of raw leachate and each treated water sampled in Dec. 2016. Sample numbers from ① to ④ correspond to the above mentioned solutions, respectively. The color phase of the treated water after fenton/PAC treatment (③) was relatively dark yellow and a slight amount of suspended solids was contained. Further improvement was observed through the retention in last reservoir (④), because of the precipitation of these suspended solids and/or dilution by rainfall. As a result, the discharge criteria in Vietnam were met in the final discharged water in Damai site, and however, it has also shown that some extent of purification is depending on the last reservoir.
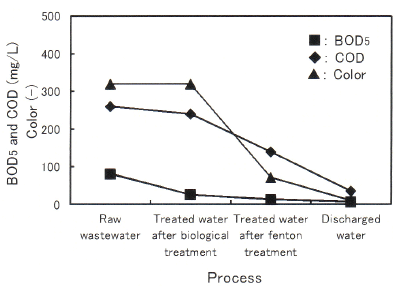
Figure 5: Variation of water quality parameters in raw leachate and each treated water in Dec. 2014.

Photo 3: Appearance of raw leachate (①) and each treated water (②~③) in Dec . 2016 .
In order to investigate the extent of the present decrease of organic compounds in Damai site, the removal rates by biological treatment and fenton/PAC treatment were calculated in each sampling water. Figures 6 and 7 shows the removal rates by biological treatment and fenton/PAC treatment, respectively. Figure 6 shows both the big variation in the extent of the decrease and the low BOD5 removal rate between 16 and 67%. Taking into account that BOD5 removal of more than 90% can be obtained under general activated sludge method, BOD5 removal rate in Damai site is very low, and there will be a room for improving the decrease of organic compounds including BOD5 components. Figure 7 also shows the big variation in fenton/PAC treatment as well as biological treatment.
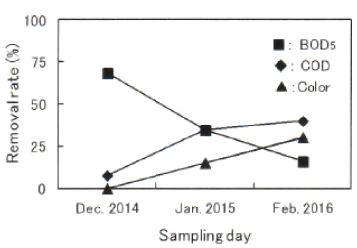
Figure 6: Removal rates by biological treatment.
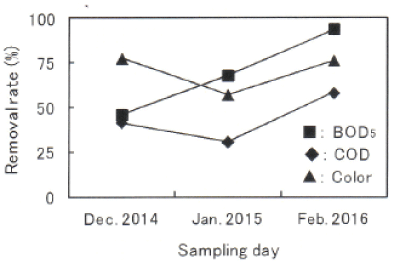
Figure 7: Removal rates by fenton/PAC treatment
It was shown from the more detailed survey that there will be a room for improving the decrease of organic compounds. Therefore, some experiments of 1-F, 1-0, 2-F, and 2-0 shown in Table 2 and Figure 3 were further conducted in lET. The experiments of 1-F and 1-0 are conducted mainly for re-estimation of the present state in Damai site, and those of 2-F and 2-0 for improvement of the decrease of organic compounds.
Experiment number |
Purpose |
Reaction condition |
|||||||
|
|
Coagulation PAC |
Biological Treatment |
Fenton Treatment |
O3 /H2 O2 |
||||
|
|
|
MLSS (Mg/L) |
HRT (h) |
FeSO4 |
H2O2 (30%) (mg/L) |
Gas flow |
H2O2 mL/1.5L |
Reaction time (min) |
Reference |
- |
400~600 |
2,000~3,000 |
20 |
400 |
150 |
- |
- |
- |
1-F |
Re-estimation of present state in Damai site |
500 |
2500 |
20 |
400 |
150 |
- |
- |
- |
500 |
2500 |
20 |
- |
- |
4 |
1.6 |
100 |
||
2-F |
Improvement of present state in Damai site |
500 |
2500 |
40 |
400 |
100 |
- |
- |
- |
500 |
2500 |
40 |
- |
- |
4 |
1.6 |
60 |
||
Table 2: Reaction conditions in new treatment system
Figure 8 shows the change of BOD5 (A), COD (B), and color (C) by these new treatment system. As shown in Figure 8A, higher extent of BOD5 removal was obtained by extending HRT from 20 to 40 h. As a result, final BOD5 values in 2-0 and 2-F met the discharge criteria in Vietnam, but not in 1-F and 1-0. These results show that the extension of HRT contributes to the increase of BOD5 removal. On the other hand , the extents of COD and color removal were almost the same extents between 1-F, 1-0, 2-F, and 2-0, as shown in Figures 8B and C, showing that the extension of HRT causes no obvious effect on the removals of COD and color.
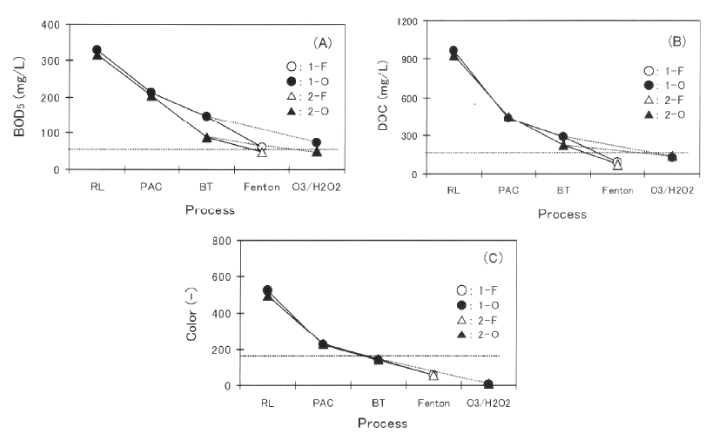
Figure 8: Change ofBOD5 (A) , COD (B), and color (C) by new treatment system.
Process; RL: raw reachate , PAC: coagulation using PAC, BT: biological treatment,
Fenton: fenton treatment, 03/H202 : ozonation with hydrogen peroxide.
Horizontal dotted line: discharge criteria in Vietnam.
The change of weight ratio of Fe to H2 O2 (O) from 2.3 to 3.4 (from 1-F to 2-F) caused a slight increase in the removal of BOD5 , COD, and these final values roughly met the discharge criteria in Vietnam, as shown in Figures 8A and B. Final color values in 1-F and 2-F met the discharge criteria in Vietnam, and however, no detectable difference between 1-F and 2-F was observed in the removal of color (see Figure 8C), These indicate that the change of weight ratio of Fe to H2 O2 (O) brings only the economic advantage of the decrease in the amount of H2 O2 , not the sufficient decrease of water quality parameters.
A decrease of BOD5 value was observed in 2-0 and final BOD5 value meet the discharge criteria in Vietnam. As shown in Figure 8C, an obvious increase of color removal was observed in 1-0 and 2-0, where O3 /H2 O2 was used instead of fenton treatment. As a result, all final values of BOD5 , COD, and color in 2-0 met the discharge criteria in Vietnam. The shortening of reaction treatment from 100 to 60 min is cost-effective, and however, treated water after 30-min reaction in 2-0 did not meet the discharge criteria of BOD5 and COD (not shown in Figure 8). These indicate that O3 /H2 O2 would be a promising method in Damai site, where H2 O2 has already used, and that the adjustment of reaction time is an important factor in terms of both the decrease of organic compounds and economical vievrpoint.
The final values of BOD5 , COD, and color obtained in 2-0 met the discharge criteria in Vietnam, and the treatment system of 2-0 shown in Table 2 and Figure 3 will be an alternative method in Damai site, especially in terms of the increase of color removal. Table 3 summarizes the characteristics of the new treatment systems of 2-0 compared with 2-F. Because the adjustment of reaction conditions such as HRT in biological treatment and reaction time in O3 /H2 O2 are very important factor, more quantitative experiments and analysis will be necessary so far for searching an optimum condition.
| Treatmentsystem | Advantage |
Disad vantage |
|
2-F |
Effective ness in BOD5 and COD removal No generation cost of ozone |
Ineffectivenes in color removal PH adjustment Sludge treatment |
|
2-0 |
Effectiveness in BOD5 , COD and color removal No pH adjustment No sludge treatment |
Generation cost of ozone |
Table 3: Characteristics of the new treatment systems
In this study, the more detailed surveys based on the previous report [6] were further conducted to estimate the present extent of the decrease of organic compounds in Damai site. In parallel of these surveys, some experiments were also conducted in lET for re-estimating the present extent of the decrease of organic compounds in Damai site, and afterward, a plan for improving the decrease of organic compounds was proposed and O3 /H2 O2 was also conducted as an alternative method of fenton treatment. The following conclusions were obtained:
The surveys and experiments were conducted as a part of AIST Water Project targeted to Asian countries. We also thank the operation stuffs in Damai site for supplying sampling water and Dr. Van from Thai Nguyen University for arranging sampling schedule.
Download Provisional PDF Here
Article Type: Research Article
Citation: Takahashi N, HibinoT, Kiyono F, Ichikawa H, Trinh Van T, et al. (2017) Survey of Damai Landfill site in Vietnam and Proposal for Improving Decrease of Organic Compounds. Int J Water Wastewater Treat 3(1): doi http://dx.doi.org/10.16966/2381-5299.136
Copyright: © 2017 Takahashi N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access