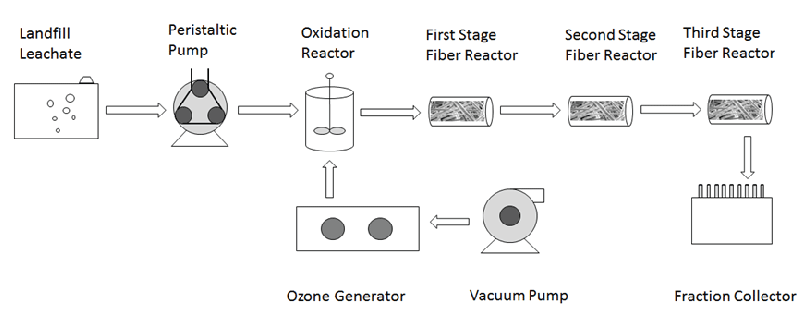
Figure 1: Experimental Setup


Boya Wang* Peter Grasel2 Owete Owete2 John Hallas3 Hafiz Ahmad4 Gang Chen1*
1Department of Civil and Environmental Engineering, FAMU-FSU College of Engineering, Tallahassee, Florida, USA*Corresponding author: Gang Chen, Department of Civil and Environmental Engineering, FAMUFSU College of Engineering, Tallahassee, FL, USA, Tel: 850-4106303; E-mail: gchen@eng.fsu.edu
Many of the landfills may contain xenobiotic compounds including monoaromatic hydrocarbons and halogenated hydrocarbons, which are well documented to have negative effects in the aquatic environment and are resistant to biodegradation. In this research, landfill leachate was treated by combined advanced oxidation and suspended fiber biofiltration. Advanced oxidation destructed xenobiotics in the landfill leachate and the following biofiltration decomposed the organic compounds. With advanced oxidation, ferrous iron in the landfill leachate was oxidized to iron oxide and coated the suspended fiber, which subsequently removed the phosphorous from the landfill leachate by adsorption. Through advanced oxidation, 76% of the organics was removed. In the following series of biofilters, organics were further removed with the effluent from the third stage biofilter meeting the discharge requirements. For iron removal, advanced oxidation only contributed to iron transformation and the following three stages of biofitration removed iron by 99%. Phosphorous was also effectively removed in this system through adsorption to the iron oxide-coated suspended fiber. This technology has potential markets for small landfills that serve low population areas and landfills that are at a distance from sewage treatment systems. It provides a sustainable and profitable solution for landfill managers to handle landfill leachate with high xenobiotic, organic, iron and phosphorous contents at the landfill sites.
Advanced oxidation; Xenobiotic; Iron; Phosphorous; Landfill
Many of the landfills might have received xenobiotic organic compounds (XOCs) from the time period when there were fewer restrictions on the disposal of hazardous waste in municipal solid waste (MSW) landfills [1]. The most frequently found XOCs in these landfills are the monoaromatic hydrocarbons (i.e., benzene, toluene, ethylbenzene and xylenes, etc.) and halogenated hydrocarbons (i.e., tetrachloroethylene and trichloroethylene, etc.). XOCs in the landfill leachate also include synthetic organochlorides such as plastics and pesticides, naturally occurring organic chemicals such as polyaromatic hydrocarbons (PAHs), and some fractions of crude oil and coal, etc. [2,3]. The structure of xenobiotic molecules is usually characterized by “unphysiological” substituents and stable chemical bonds, making xenobiotics resistant to degradation. These pollutants are well documented to have negative effects in the aquatic environment [4].
The composition and concentration of XOCs in the landfill leachate are influenced by the type of deposited waste, the quality of the refuse, the hydrogeological factors, and the age of the landfill [3]. Although biological treatment is cost-effective when compared to other treatment options, it is not efficient when treating leachate from mature landfills that contain recalcitrant and/or non-biodegradable organic substances such as XOCs. However, when combined with advanced oxidation, which can convert the initially persistent organic compounds into more biodegradable intermediates, biological treatment processes have shown the promising future [5]. Advanced oxidation processes have demonstrated the capacity of degrading the recalcitrant XOCs. Therefore, advanced oxidation processes, i.e., the usage of oxidizing chemicals such as ozone (O3 ), hydrogen peroxide (H2O2) and UV radiation, etc. can be used as alternatives to biodegradation to effectively break the stable xenobiotic structures in landfill leachate [6]. In practice, the use of O3 , H2O2 , UV light and other oxidizing compounds to degrade recalcitrant organic compounds has evoked interest during the last couple of years [7,8]. By destructing the xenobiotic organic matters, advanced oxidation increases the biodegradation of landfill leachate for the subsequent bioassociated treatment [9-11]. During advanced oxidation, hydroxyl radicals of • OH are produced, which contain a very high electronic potential and can oxidize a variety of organic compounds. The versatility of advanced oxidation is enhanced by the fact that they offer different possible ways for the production of • OH radicals, thus allowing a better compliance with the specific treatment requirements [12]. The difficulty of advanced oxidation in the practical application is the choice or design of the most efficient oxidation system for the given pollutants. Advantages and disadvantages of the usage of specific oxidizing agents are ascertained.
Advanced oxidation processes are able to oxidize organic substances to their highest stable oxidation states (i.e., complete mineralization) and improve the biodegradability of recalcitrant organic pollutants up to a value compatible with the subsequent economical biological treatment [13]. These processes are commonly evaluated in terms of COD, BOD, and BOD/COD. Initially, ozonation was the first to demonstrate to have the effectiveness in eliminating COD (about 50 to 70% reductions in most cases) of the wastewater effluent [14]. However, the efficiency of ozonation on the oxidation of stabilized leachate has not been consistent. For some cases, only 30% COD depletion was observed after 1 hour of ozonation with an ozone consumption of 1.3 to 1.5 g O3 /g COD [3]. On the other hand, COD removal was dramatically increased when ozonation was combined with other advanced oxidation means such as hydrogen peroxide. The removal efficiency as high as 90% was achieved for the O3 / H2O2 process [15,16]. Concerning the H2O2 /UV process, the BOD5 / COD ratio also increased significantly, from 0.1 to 0.45 [17]. Fenton and photo-Fenton processes can decrease COD by 45 to 75% and 70 to 78%, respectively. In term of biodegradability improvement, BOD5 /COD ratios close to 0.5 after oxidation have been reported by the Fenton process [18]. Under acidic conditions, the Fe2+/H2O2 mixture produces • OH radicals in a very effective way. It was estimated that the cost of O3 /H2O2 system was about $2.3per kg COD [19,20] and the ozone-granular activated carbon adsorption was $2 to $4 per m3 [21]. On the other hand, the estimated operational cost for the Fenton treatment of landfill leachate was $5.19 per m3 [22]. It should be noted that the Fenton’s process requires low pH, which limits its wide applications [23,24].
As an innovative technology for wastewater treatment, the suspended fiber biofiltration makes biological contact oxidation possible, which can significantly improve BOD and COD removal and decrease the sludge yield. For iron removal, contact oxidation is achieved by microbial mediated iron oxidation and fixation during which ferrous iron is oxidized to ferric iron and fixed onto the filter media [25]. Consequently, there is minimal ferric iron suspending in the solution that can escape the filter. When suspended fiber biofiltration is used for the landfill leachate treatment, iron and phosphorous can be removed in series. Specifically, iron is first oxidized by contact oxidation if advanced oxidation is included and coats the suspended fiber in the form of iron oxides, which has high binding strength for phosphorus [26,27]. The prominent correlation between phosphorus and iron oxides has led to numerous examinations of phosphorous adsorption capacities and mechanisms by iron oxides. The mechanism of phosphorous adsorption onto iron oxides is generally dominated by ligand exchange in which two singly coordinated hydroxyl groups or water molecules are replaced by a single phosphate anion, resulting in the formation of a bidentate, binuclear complex [28,29]. The phosphate surface complexes are very stable, resulting in slow exchange rates and an apparent irreversibility (hysterisis) of phosphorus adsorption [30,31]. Since the suspended fiber biofilter has dramatically increased surface areas, phosphorous removal can thus achieve a significantly high efficiency.
In this research, advanced oxidizing processes, i.e., the usage of UV or oxidizing chemicals were introduced to the leachate treatment and suspended fiber biofiltration for the degradation of xenobiotic compounds and removal of phosphorous from landfill leachate. Besides xenobiotic destruction, advanced oxidation also led to improved aerobic biodegradation of organics and iron and phosphorus removal. After xenobiotic destruction by advanced oxidation, iron, phosphorous and organics were removed step by step by a series of suspended fiber biofilters. The combination of advanced oxidizing processes, i.e., the usage of oxidizing chemicals and the subsequent suspended fiber biofiltration provides a novel and cost- and space-saving treatment alternative for handling landfill leachate at landfill sites.
Landfill leachate was collected from Springhill Landfill, located in Campbellton, Florida. Springhill Landfill currently accepts domestic wastes from Leon County through the transfer station at the Gum Road. In addition, Springhill Landfill offers the following non-hazardous waste disposal services: asbestos-friable, asbestos-non-friable, auto shredder fluff, biosolids, construction and demolition debris, drum managementliquids, drum management-solids, industrial and special waste, liquifix (solidification services), municipal solid waste, tires, yard waste and CERCLA waste. The leachate was collected in temperature-controlled containers at 4°C and transported to the laboratory immediately. BOD5 and COD were characterized in the laboratory following the standard methods and NH4+-N concentrations were quantified colorimetrically by means of spectrophotometry [32]. The collected landfill leachate had a BOD5 in the range of 346 to 512 mg/L, COD in the range of 1,278 to 1,767 mg/L, and NH4+-N in the range of 416 to 674 mg/L. It should be noted that these measurements were based on the supernatant of the collected samples. To analyze the components of the solid precipitates, EDX analysis was conducted and the results showed that the leachate solid precipitate had 74% C, 16% O, 9% Si and 1% Fe. The characteristics of the landfill leachate used for this research are summarized in Table 1.
Component |
Range (mg/L except for pH) |
Average |
pH |
6.97 - 7.84 |
7.41 |
TSS |
1,645 - 1,897 |
1,771 |
COD |
1,278 - 1,767 |
1,522 |
BOD5 |
346 - 512 |
428 |
TKN |
1,045 - 1,879 |
1,462 |
Ammonia-N |
416 - 674 |
542 |
Nitrate-N |
46.8 - 124.6 |
62.3 |
Alkalinity (as CaCO3) |
2,141 - 5,264 |
3,702 |
Total Phosphate |
168 - 326 |
247 |
Sulphate |
156 - 246 |
201 |
Chloride |
1,897 - 3,246 |
2,571 |
Sodium |
1,246 - 2,874 |
2,060 |
Calcium |
167 - 208 |
187 |
Magnesium |
115 - 462 |
288 |
Cadmium |
0.01 - 0.05 |
0.03 |
Copper |
0.46 - 0.64 |
0.55 |
Iron |
156 - 276 |
216 |
Manganese |
10.5 - 16.7 |
13.6 |
Table 1: Characteristics of the Landfill Leachate
Landfill leachate was treated by advanced oxidation in the oxidation reactor, after which the leachate was further treated in a series of suspended fiber (polypropylene) biofilters (Figure1). The oxidation reactor had a dimension of 2 cm (diameter) × 8 cm (length) and the suspended fiber biofilters had a dimension of 4 cm (diameter) × 15 cm (length) each. The flow rate was 0.4 mL/min and the subsequent retention time of the oxidation reactor and the following suspended fiber biofilters was 62 min and 235 min. Leachate oxidation by advanced oxidation was first investigated separately in batch experiments. Ozone and hydrogen peroxide were applied at an ozone/COD or hydrogen peroxide/COD molar ratio up to 1.0. UV radiation was applied in the range of 10 to 100 mJ/cm2.

Figure 1: Experimental Setup
Inside the first stage suspended fiber biofilter, the fiber was coated by iron oxides through contact oxidation after advanced oxidation. The iron-coated fiber had a strong binding strength for phosphorus. The second and third stage fiber biofilters were inoculated with bacterial consortia that were responsible for organic decomposition and contact oxidation. Landfill soil was used as inocula for the consortia cultivation. It was found that both gram negative bacteria (57%) and gram positive bacteria (43%) were present in almost equal frequencies in the consortia. Bacillus dominated the generic composition by 25%, which was followed by Vibrio (17%). Dominance of Bacillus was in agreement with the findings by Krishnan and Saramma [33]. The other genera resembled the Enterobacteriaceae group, Arthrobacter, Brevibacterium, Aeromonas, and Pseudomnas, etc. During advanced oxidation, xenobiotics were destructed and the byproducts and residuals were further degraded within the suspended fiber biofilters by the inoculated microorganisms.
For this research, advanced oxidation of the leachate was evaluated using ozone, hydrogen peroxide and UV radiation. Ozone was produced from oxygen using a 4 g/hr ozone generator (RMU 16-04, Azcozon Industries, Langley, B.C. Canada V1M 3E5, Canada). This ozone generator was capable of producing up to 4 g/hr of ozone gas using compressed oxygen with glass/quartz electrodes. Fresh ozone stock solution was generated by bubbling O3 gas (1.5%-3%) into a 250 mL ozone gas washer with 10 mM sodium phosphate buffered at pH 6. To minimize the decay of ozone in the stock solution, the ozone washer was maintained at <6°C. Ozone concentration was determined following the standard method (4500-O3 B Indigo Colorimetric Method) [34]. The thus measured ozone stock solution concentration was 33 mg/L O3. UV radiation was achieved by exposing under a UV collimated beam, generated using 4 germicidalUV lamps (15 W each) emitting at λ=254 nm (G15T8, General Electric Co., Cleveland, OH). The UV absorbance was determined by the Cary 60 UV-VIS spectrophotometer. The incident light intensity for each sample was measured by an IL 1700 radiometer equipped with a SED240 sensor (International Light, Peabody, MA, U.S.A.). The corresponding UV dose was calculated by multiplying the radiation time by the intensity of the UV lamp measured at the wavelength of 254 nm. H2O2 was obtained directly from Sigma-Aldrich.
High-pressure liquid chromatography (HPLC), operating in reversephase was used to measure the concentration of pCBA and BPA with a wavelength of 234 nm for the UV detector and a retention time of 21 min and 17 min respectively. For ferrous iron quantification, 1,10-Phenanthroline Method was utilized [35]. In the presence of 1,10-phenanthroline (C12H8N2- H2O), ferrous iron formed a stable, orange-colored complex with the reagent:
$${\rm{F}}{{\rm{e}}^{2 + }}(aq) + 3(ph){{\rm{H}}^ + } = {\left[ {{\rm{Fe(ph}}{{\rm{)}}_3}} \right]^{2 + }} + 3{{\rm{H}}^ + }\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(1)$$
For this experiment, 0.0125 M 1, 10-phenanthroline was used and ferrous iron concentrations were quantified using the spectrophotometer (Shimadzu UV-1650 PC) at a wavelength of 520 nm. For phosphorus quantification, the ascorbic acid method was used.
NMR analysis was performed using a Varian Unity 600-MHz instrument at 298 K. About 50 mg of the samples was dissolved in 1 mL of deuterium oxide (D2O, Sigma, 99.9 % atom D). 1H-NMR spectra were recorded at 600 MHz under the following conditions: 1.7 sec acquisition time, 1.0 sec relaxation delay and pulse width of 45°.
Both COD and BOD5 values were found to decrease with the increase of the ozone dosage when the landfill leachate was ozonated at different ozone dosage/COD molar ratios (Figure 2). However, the BOD5 /COD value increased with enhanced oxidation, which would be beneficial for the following biological degradation processes. It was suspected that through ozonation, some recalcitrant organic compounds were broken into simple organic compounds that can be degraded biologically. The ozonation of landfill leachate was monitored by HPLC to observe the decomposition dynamics. The major byproducts were found to include hydroquinone, catechol and simple organic acids such as maleic acid and several unidentified compounds. Recalcitrant accumulated product of oxalic acid (C2H2O4 ) was also discovered during ozonation. Oxalic acid increased from 21 mg/L at the beginning to 45 mg/L after 50 minutes’ ozonation (Figure 3). Biodegradation of the final products, a mixture of oxalic and formic acids with a concentration of 100 mg/L of each was further investigated. It was discovered that microorganisms were able to eliminate both compounds within two days. These results demonstrated the promising application potentials of ozonation for the treatment of landfill leachate, i.e., highly toxic substrates that cannot be eliminated by bioprocesses can be degraded by ozonation and transformed into compounds that can be biologically decomposed. The consortia that were responsible for oxalic and formic acid degradation were the ones cultured and inoculated for the suspended fiber biofilters. They showed the capability to eliminate the ozonation products.
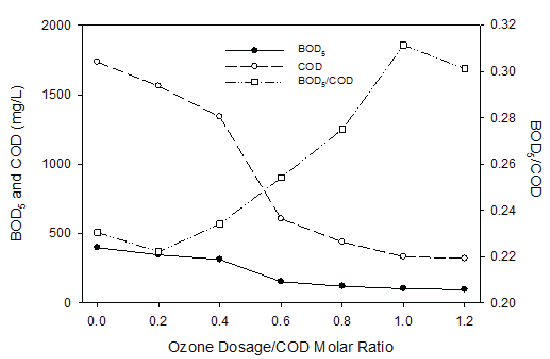
Figure 2: BOD5 /COD Ratio as a Function of Ozonation
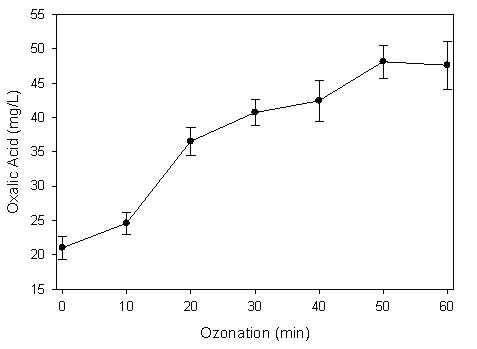
Figure 3: Oxalic Acid Accumulation after Ozonation
Bisphenol A (BPA), a plastic additive, is used in the production of polycarbonate plastics and epoxy resins. Studies have shown that significant amounts of BPA can leach from plastics and enter the environment including the landfill leachate, creating a public health concern. As a xenobiotic organic compound (XOC), the concentration of BPA in the leachate varies depending on the age and composition of the waste in the landfill. The typical BPA concentration in the landfill leachate is in the range of 0.07 to 269 μg/L. The conventional biological treatment of landfill leachate is not efficient in removing BPA. In this research, BPA removal was found to increase with the increase of ozone dosage, which was also a function of pH (Figure 4). At ozone dosage of 10 mg/L and pH 9.5 with a reaction time of 10 minutes, 60% of BPA can be removed. Oxidation kinetics of BPA in landfill leachate was also investigated in this research with an ozone dosage of 8.9 mg/L. BPA depletion increased with the reaction of ozonation for all the pH conditions investigated in this study. However, the BPA depletion with ozonation was more pronounced at pH 7 and 9 as compared to that of pH 5 (Figure 5). It seemed that BPA depletion was more effective by ozonation after five minutes. Similarly, after 10 minutes’ ozonation, the depletion became moderate again. In a separate experiment, BPA depletion using combined ozone and hydrogen peroxide was also tested. The results showed that applying ozone alone was more efficient in BPA depletion than combined ozonation with hydrogen peroxide.
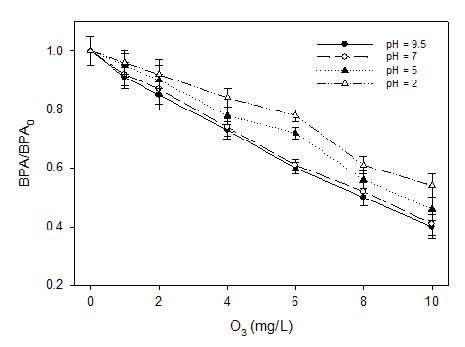
Figure 4: BPA Removal by Ozonation as a Function of pH
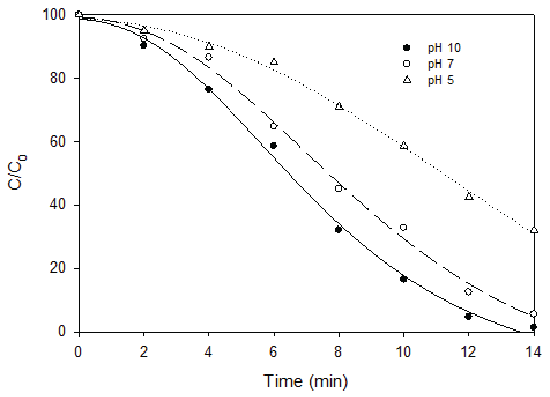
Figure 5: BPA Depletion Kinetics by Ozonation
COD removal was found to increase with the increased H2O2 concentration for both pH levels when the landfill leachate was reacted with hydrogen peroxide at different H2O2 /COD mole ratios at pH 7.4 and 9.6 (Figure 6). COD removal was better observed for pH 7.4 than that of 9.6. Compared to ozonation, hydrogen peroxide was more effective in removing COD from the landfill leachate. For instance, when hydrogen peroxide was applied at a H2O2 /COD mole ratio of 0.2, 0.5 and 1.0, 72-75%, 75-80% and 80-90% COD removal were achieved. On the contrary, around 40%, 50% and 70% COD removal were observed with corresponding ozone/COD molar ratios of 0.2, 0.5 and 1.0. BOD5 values were also found to decrease with the increase of the hydrogen peroxide concentration (Figure 7). However, the BOD5 /COD value increased with increased hydrogen peroxide dosage, which again would be beneficial for the following biological degradation processes. Compared to ozonation, hydrogen peroxide depleted COD more rapidly at low hydrogen peroxide dosages. For instance, at H2O2 /COD molar ratio of 0.4, 70% of COD was depleted. However, at ozone/COD ratio of 0.4, only 25% of COD was depleted. On the other hand, at high dosages, similar COD depletions were observed for both hydrogen peroxide and ozonation. At hydrogen peroxide/COD or ozone/COD molar ratio greater than 0.8, COD depletion became moderate for both hydrogen peroxide application and ozonation. It should be noted that the high removal of COD by the usage of H2O2 might be attributed to the Fenton reaction and subsequent Fe(OH)3 precipitation, which resulted in the organic removal by means of coagulation. This means that the organic decrease was by means of coagulation instead of oxidation.
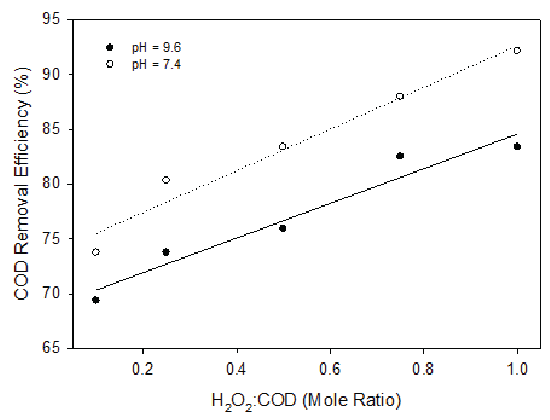
Figure 6: COD Removal with Variable H2O2 Dosages at pH 7.4 and 9.6
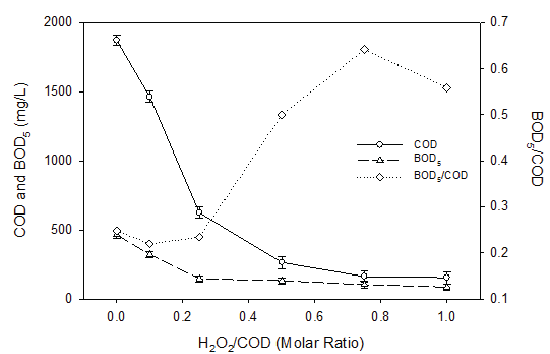
Figure 7: BOD5 /COD Ratio as a Function of H2O2 Dosage
UV radiation in the range of 10 to 100 mJ/cm2 (by varying the exposure time from 0 to 15 min) was applied to the landfill leachate at pH 4.0, 7.4 and 9.6. The results demonstrated UV radiation had better COD removal effects at high and low pH values than that of neutral pH (Figure 8). However, the removal efficiency was low for all the tested pH levels. It is therefore recommended that UV not be used alone for the treatment of landfill leachate. When H2O2 and UV radiation were combined, high COD removal efficiency was observed at low H2O2 dosage (H2O2 /COD=0.25), especially at low and high pH values (Figure 9). At low and high pH and H2O2 dosage of H2O2 /COD=0.25, COD removal increased with the increase of UV radiation, reaching 96% for pH 4.0 and 90% for pH 9.6. At pH of 7.4, around 75% of COD can be removed and variation of UV radiation showed minimal effect. In other experiments, UV radiation combined with ozonation was also studied and the results were not satisfactory.
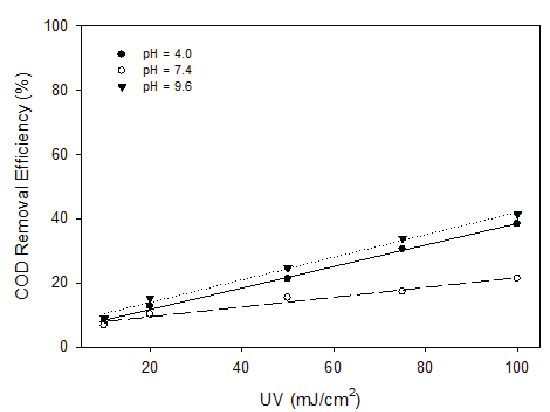
Figure 8: COD Removal with Variable UV Radiation at pH 4.0, 7.4 and 9.6
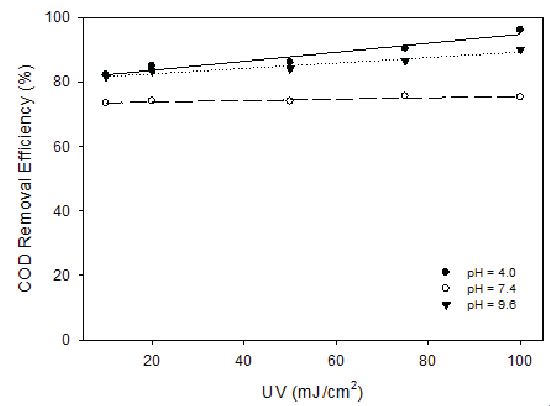
Figure 9: COD Removal with Variable UV Radiation When Combined with H2O2 at Dosage of H2O2/COD = 0.25 and pH 4.0, 7.4 and 9.6
Ammonium nitrogen was oxidized to nitrate by advanced oxidation (Figure 10). Once in the nitrate format, nitrogen was not further removed in the system since the system was operated under oxidized conditions.
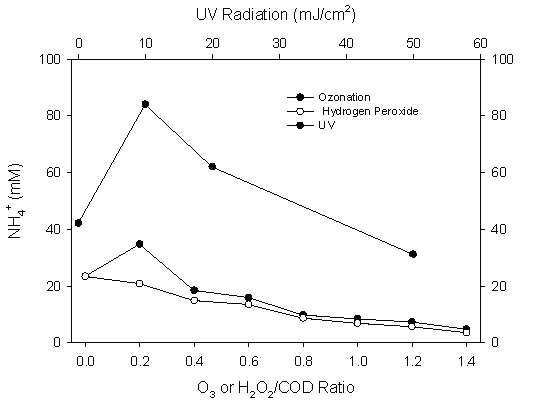
Figure 10: Ammonium Oxidation by Different Advanced Oxidation Means
After advanced oxidation, the treated leachate was introduced to the series of fiber biofilters. Inside the biofilters, inoculated microbial consortia strategically positioned themselves on the suspended fiber to form a biofilm. For advanced oxidation treatments followed by corresponding suspended fiber biofiltration, ozonation combined with hydrogen peroxide application had the best treatment results, i.e., COD was reduced from 1,750 mg/L to 70 mg/L (Figure 11). UV radiation alone had the lowest COD removal observations, which could only reduce the COD to 200 mg/L after oxidation and three stages of biofiltration. Iron removal is very important for the landfill leachate treatment in Northwest Florida owing to the high iron content in the soil. During landfill operation, Fe3+ is reduced to Fe2+ and released to the leachate. Within the biofilters, iron fixing bacteria can oxidize ferrous iron to ferric iron, even under oxygen poor conditions by extracting carbon dioxide from ferrous bicarbonate and utilizing iron bearing organic acid complexes as the carbon source. For an input iron concentration around 100 mg/L, an effluent iron concentration of 1 mg/L can be achieved at the end of the third stage biofiltration. For phosphorous removal, iron oxide-coated on the fiber surfaces played the key role, on which phosphorus was adsorbed (Figure 12). For phosphorus to adsorb to iron oxide surfaces, phosphorus replaced singly coordinated OH- groups and then reorganized into a very stable binuclear bridge between the cations. This sorption process was coupled with the release of OH- ions, thus this process was favored by low pH values [36]. This is because phosphorous sorption process released OH- , increasing the solution pH, which prevented Fe3+ hydrolysis process that also preferred low pH.
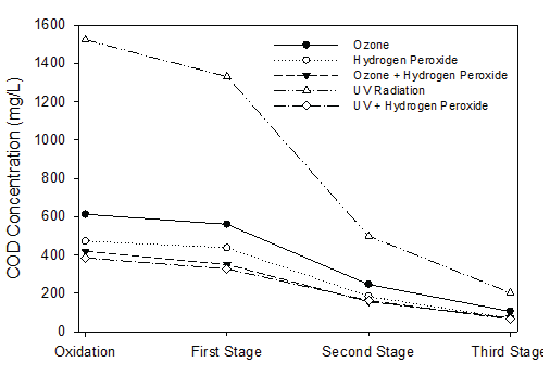
Figure 11: Comparison of COD Removal by Different Advanced Oxidation Means
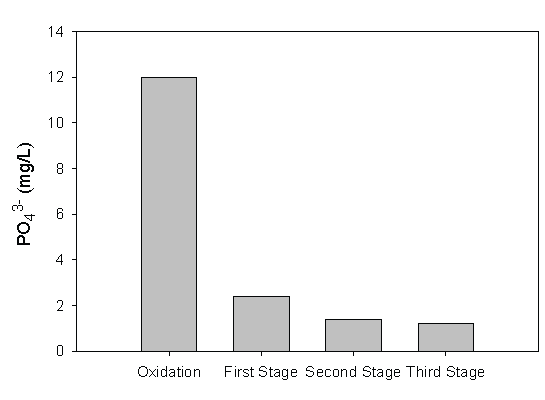
Figure 12: Phosphorous Removal with Ozone as the Advanced Oxidation Means
Observations of this research were consistent with the results of prior research. For instance, COD removal of 50 to 70% for wastewater treatment and 85% for landfill leachate was reported [37,24]. In this research, 65.0% to 81.4% removal was observed with ozone/COD ratios of 0.6 to 1.2. With ozonation, BOD5 /COD ratio increased to 0.3 with ozone/ COD molar ratio of 1.0. In another study, BOB5 /COD ratio of 0.3 was also observed at pH 9 when 1800 mg O3 was applied to a landfill leachate with a COD value of 1880 mg/L [38]. Similar BPA removal was also observed with prior research during ozonation [17]. After advanced oxidation, the overall treatment efficiency of the system was also comparable with prior research (96% versus 90% total COD removal) [39].
The characteristics of landfill leachate are affected by many factors, such as age, precipitation, weather variation, and waste types and compositions. Owing to the complicated composition and processes, besides aminoacids, carbohydrates and carboxylic acids which are generally present in landfill leachate, recalcitrant xenobiotic organic compounds are commonly found in the landfill leachate. To address landfill leachate’s heterogeneous and undefined character, advanced oxidation processes (AOPs) have demonstrated the effectiveness in eliminating organics and xenobiotics. Traditionally, chemical oxidation has been widely used for the treatment of effluents containing refractory compounds such as landfill leachate. Currently, AOPs are drawing more and more attention, especially the AOP processes that use a combination of strong oxidants, e.g. ozone, hydrogen peroxide and UV radiation, etc. These AOP processes have shown great enhancement on organic removal and xenobiotic destruction. Practically, AOPs have been adapted to old and well-stabilized landfills to oxidize organic substances to their highest stable oxidation states (complete mineralization) and/or improve the biodegradability of recalcitrant organic pollutants up to a value compatible with subsequent economical biological treatment. In the term of biodegradability improvement, BOD/ COD ratios have been found to increase after oxidation.
Common drawbacks of AOPs are the high demand of energy costs as compared to that of the relatively low biological treatment processes. In addition, complete degradation (mineralization) of the pollutants requires high oxidant doses. Furthermore, some intermediate oxidation products can actually raise the toxicity of the leachate. To further evaluate the advanced oxidation processes, the UV spectrum of the treated leachate was further analyzed. UV–VIS spectra of the treated samples were obtained with the spectrophotometer within the wavelength range of 200 to 800 nm. The E2/E3 and E4/E6 ratios (parameters that are inversely related to molecular weight and aromaticity, and proportional to the O, C, and carboxyl group content and total acidity) were determined by measuring the absorbance at 254 and 365 nm for E2/E3 ratio and at 465 and 665 nm for E4/E6 ratio. In general, the UV–VIS spectra exhibited an exponential decline with increasing wavelength. The absorbance at λ<250 nm might result from the absorption of radiation by the double bonds, especially the aromatic C=C and ketonic C=O functional groups of aromatic chromophores and/or other organic compounds.
NMR analysis demonstrated that 1H-NMR spectra included the aliphatic protons region, a broad resonance assigned to protons on carbons attached to O or N atoms (carbohydrate) and the aromatic, phenol, and carboxylic proton region. Different advanced oxidation resulted different chemical shift values, which differed in intensity and linewidth. In general, advanced oxidation led to decreased aromatic, phenol and carboxylic proton and increased carbohydrate contents (Figure 13). The NMR of the fiber that are used in the biofiltration was also conducted before and after the leachate treatment (Figure 14). The aromatic carbohydrate proton region and the aliphatic region were minimal for the fiber before the leachate treatment. However, after the leachate treatment, the spectra showed a highly resolved signal arising from aliphatic acids. A relatively high amount of aromatic proton was also observed, indicating that aromatic structures accumulated in the fiber during the leachate treatment. In the regions of carbohydrates and proteins, there was obvious increase in the resonance intensity, indicating sugar-like component accumulation. The resonance signals that attributed to CHOH and CH2OH functional groups (i.e., polysaccharides moieties) as well as aminomethine groups [−CH(NH−)] and methylene groups that were bonded to amide functional groups [−CH2(NHCO−)] were also observed. These observations demonstrated that advanced oxidation contributed to the destruction of xenobiotics and the released lowmolecular weight compounds had a chance to accumulate in the fiber media and be decomposed by the microorganisms in the system. In the aliphatic region, the peaks were believed to be attached to aliphatic carbons (methyl or methylene groups), which were attached to functional groups such as carboxyl group or aromatic ring. The intensity of the signals for the aliphatic, aromatic, and carboxyl groups tended to vary, depending on the origin and type of humic matters. These results are consistent with those obtained previously from the UV–VIS spectra.
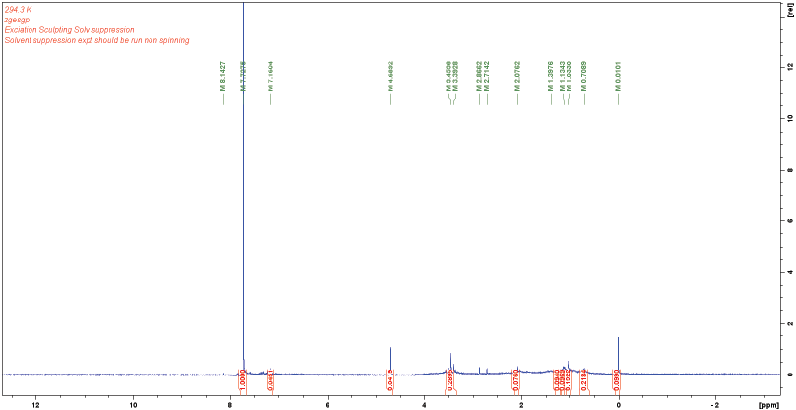
Figure 13: NMR Spectra of the Landfill Leachate
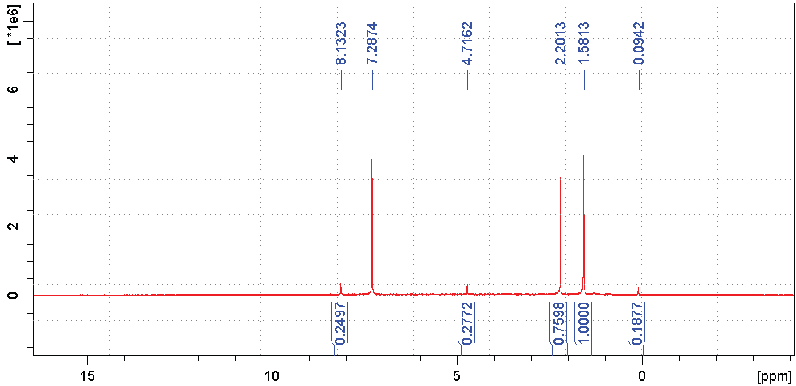
Figure 14: NMR Spectra of the Suspended Fiber after Leachate Treatment
Iron is one of the most abundant metals of the Earth’s crust. It occurs naturally in water in soluble form as the ferrous iron (bivalent iron in dissolved form Fe2+ or Fe(OH)+) or complex form like the ferric iron (trivalent iron of Fe3+ or precipitated as Fe(OH)3). Nearby landfills in Northwest Florida, owing to the high iron contents in the soil, elevated iron concentrations are commonly observed. In general, iron does not present a danger to human health or the environment, but it brings unpleasantness of an aesthetic and organoleptic nature. Indeed, iron gives a rust color to the water, which can stain linen, sanitary facilities, or even food industry products. Iron also gives a metallic taste to water, making it unpleasant for consumption. It can also be at the origin of corrosion in drain sewers, due to the development of microorganisms, the ferrobacteries. However, treated leachate must meet the drinking water standard of 0.3 mg/L total iron before being discharged to the receiving water bodies.
Ferrous iron is soluble as a cation, while ferric iron is not. For the natural removal of dissolved iron, the redox potential of the water promotes the oxidation of ferrous iron to ferric iron, which can precipitate in the form of ferric iron hydroxide, Fe(OH)3 :
$${\rm{F}}{{\rm{e}}^{2 + }}\buildrel {{\rm{Oxidation}}} \over
\longrightarrow {\rm{F}}{{\rm{e}}^{3 + }}\buildrel {{\rm{Precipitation}}} \over
\longrightarrow {\rm{Fe(OH}}{{\rm{)}}_3}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(2)$$
Once ferrous iron is oxidized, hydrolysis proceeds:
$$\left[ {{\rm{F}}{{\rm{e}}_{\rm{m}}}{{({\rm{OH}})}_{\rm{X}}}} \right]_{\rm{n}}^{(3m - X)n + } + {\rm{yO}}{{\rm{H}}^ - } \to \left[ {{\rm{F}}{{\rm{e}}_{\rm{m}}}{{({\rm{OH}})}_{{\rm{X + (y/n)}}}}} \right]_{\rm{n}}^{\left[ {3m - X - ({\rm{y/n)}})} \right]n + }\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(3)$$
The oxidation kinetics of ferrous iron is well known to be pH dependent, with the slow oxidation kinetics of ferrous iron at low pH. This is important for microbial-mediated iron oxidation and fixation. Many known iron-oxidizing microorganisms in the environment can also oxidize ferrous iron at neutral pH. Since the abiotic oxidation of iron is very fast, these microbes must compete effectively with the abiotic process. Additionally, microbes must compete with each other for the available ferrous iron. The organisms that are able to utilize the iron faster in the particular environment make up the predominant part of the iron oxidizing community. Ferric hydroxide (Fe(OH)3 ) is the direct result of ferrous iron oxidation. The principal forms include amorphous hydrous ferric oxide (Fe2O3•XH2O), maghemite (gamma-Fe2O3), lepidocrocite (gamma-FeOOH), hematite (alpha-Fe2O3), and goethite (alpha-FeOOH). With the increasing pH, the concentration of metal ions such as iron is generally low due to the decreasing solubility of many different metal ions. However, lead is an exception since it forms a very stable complex with humic acids. Besides the effect of shifting pH on metal ions, there is a possibility of sulphate reduction to sulphide, which increases the precipitation of metals ions.
Adsorption of phosphorous onto iron hydroxides is attributed to the iron hydroxides coated on the polypropylene fiber. It is believed that phosphorous adsorption on iron hydroxides is generally dominated by ligand exchange in which two singly coordinated hydroxyl groups or water molecules are replaced by a single phosphate anion [40]. Since H2O is a more mobile ligand than OH-, adsorption is therefore favored at lower pH.
It is believed that four key characteristics impact phosphorus adsorption on the iron hydroxide surfaces, i.e., the easiness of hydroxyl release, the specificity toward binding sites, hysteresis, and the surface charges [40,41]. Iron hydroxides formed under advanced oxidation conditions contribute positively for phosphorus removal in the treatment system.
The suspended fiber biofilters were operated under aerobic conditions in this research. There has also been a major impetus to investigate the ability of microorganisms to biodegrade hydrocarbons in the absence of oxygen. This is motivated by the fact that landfill leachate usually has low oxygen content. Studies of microbial degradation of monoaromatic hydrocarbons have resulted in the identification and isolation of a number of different anaerobic bacterial strains capable of degrading one or more monoaromatic hydrocarbons. Of these compounds, the anaerobic biodegradation of toluene is probably the most comprehensively understood. Toluene is biodegradable with nitrate, Mn(IV), Fe(III), humic substances, sulfate, and CO2 as terminal electron acceptors. More recently, it has been demonstrated that toluene can also be assimilated anaerobically as a carbon source by anoxygenic phototrophs. However, owing to the advanced oxidation, this design is not suitable for anaerobic processes.
From this research, it is demonstrated that landfill leachate can be treated by combined advanced oxidation and suspended fiber biofiltration. Advanced oxidation can destruct xenobiotics in the landfill leachate and the following biofiltration can decompose the organic compounds. Most importantly, with advanced oxidation, ferrous iron in the landfill leachate is oxidized to iron oxide and coat the suspended fiber, which can remove phosphorous from the landfill leachate through adsorption. Through advanced oxidation, 76% of the organics can be removed. In the following up series of biofilters, organics are further removed with the effluent from the third stage biofilter meeting the discharge requirements. For iron removal, advanced oxidation only contributes to iron transformation. However, the following three stage biofiltration can remove iron by 99% and also meet the discharge requirements. It seems that the treatment system performs better for landfill leachate with high iron contents. Finally, phosphorous can also be effectively removed in this system through adsorption to the iron oxide that coat the suspended fiber. With an input close to 12 mg/L, an effluent of 1 mg/L can be achieved, which complies with the discharge requirements.
The work was support by Hinkley Center for Solid and Hazardous Waste Management through Grant No. UF-EIES-0632020-FSU to Florida State University
Download Provisional PDF Here
Article Type: Research Article
Citation: Wang B, Grasel P, Owete O, Hallas J, Ahmad H, et al. (2016) Advanced Oxidation and Suspended Fiber Biofiltration for the Treatment of Landfill Leachate. Int J Water Wastewater Treat 2(5): doi http://dx.doi.org/10.16966/2381-5299.132
Copyright: © 2016 Wang B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access