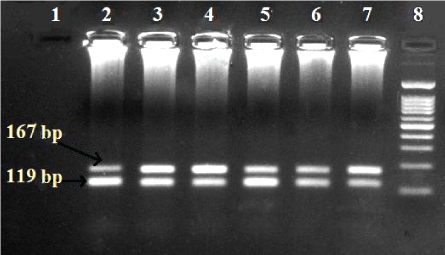
Figure 1: Agarose gel result for HBV genotypes using Multiplex nested PCR (lane 8: 100 bp ladder; lane 1: Negative control; lane 2, 3, 4, 5, 6, 7: Mixed genotypes D and E).


Mohamed O Mustafa1* Khalid A Enan1 Isam M Elkhidir2 Abdel Rahim M El Hussein1
1Department of Virology, Central Laboratory, Ministry of Higher Education and Scientific Research, Sudan*Corresponding author: Mohamed O Mustafa, Department of Virology, Central Laboratory, Khartoum, Ministry of Higher Education and Scientific Research, Sudan, Tel: +249916503948; +249-120799630; E-mail: king07_moha@yahoo.com
Background: Occult (Inapparent) Hepatitis B Virus Infection (OBI) distinguished by undetectable HBsAg and detectable anti-Hbc continues to be a great threat to blood safety. We examined the prevalence of OBI and HBV genotypes of OBI among renal transplant patients.
Methods: One hundred samples were collected from renal transplant patient from different centers in Khartoum State. The specimens were investigated for HBV Surface Antigen (HBsAg) and HBV core (HBc) antibodies using Immune Enzymatic Assay (ELISA), and HBV DNA using PCR and Real time PCR and for HBV genotypes using Multiplex Nested PCR.
Results: A total of 100 clinical samples were tested for HBsAg of which 97 samples (97%) were negative for HBsAg by ELISA and thirty-seven samples (38.1%) were positive for HBc antibodies. All of the 37 samples positive for HBc antibodies were negative for HBV DNA (0.0%) using PCR; Real time PCR detected only 19(51.4%) positive DNA samples; while different HBV genotypes (A,D, and E) and their co-infections thereof were detected in 24(64.9%) samples using Multiplex Nested PCR. The percentage of anti-HBc positivity was higher (42%) in males compared to females (33%). Occult HBV prevalence was almost equal when using Multiplex Nested PCR being 65.2% and 64.3% in males and females respectively while it was higher in females (57.1%) than in males (47.8%) when using Real time PCR.
Conclusion: The results indicated a moderate incidence of occult HBV in renal transplant patients in Khartoum, Sudan. These results also indicated that occult HBV may not be detectable using conventional PCR but can be detected by using Real time PCR and Multiplex Nested PCR. Multiplex Nested PCR test can also detect Occult HBV at higher frequency by detecting viral genotypes which cannot otherwise be detected by using Real time PCR alone. This notion is very important to avoid accidental transmission through dialysis machines or blood transfusion.
Renal transplant patients; HBV Genotypes; Occult HBV; HBsAg; Anti-HBc antibodies
Human Hepatitis B Virus (HBV) infection is a significant health problem and is a principal factor in renal and liver disease. It is postulated that over five hundred million individuals are infected with HBV worldwide and more than one million deaths are annually attributed to the effects of HBV infection [1,2].
Nowadays, renal transplant patients experience less morbidity and mortality as result of infections than during the initial decades after the advent of renal transplantation. However, infections remain significant threats to the health of renal transplant recipient, mainly because infections are associated with organ rejection. Particularly important in this respect are the viruses which can play significant roles in morbidity and mortality in renal transplant recipient [3].
Occult Hepatitis B Virus Infection (OBI) infection can be defined as a particular form of HBV infection where patients are negative for Hepatitis B Surface Antigen (HBsAg) but are anti-HBc antibodies positive, while at the same time being positive for HBV DNA in the liver and sometimes in the serum. OBI was first suspected in the 1970’s when transmission of HBV infection through blood transfusion or in renal patients occurred from donors with serology suggestive of past HBV infection but with clearance of HBsAg [4,5]. OBI may be related to the host’s immune response and/or the effect of co-infection with other disease agents [4]. Naturally occurring mutation in the promoter pre-S2/S region were detected in cases of OBI [6,7]. Later, it was observed that these mutations can cause modified HBsAg expression and an increased large-HBsAg/small-HBsAg ratio leading to reduced antigen secretion [8].
Several studies showed that different HBV genotypes are associated with different outcome e.g. HBV genotype D was connected with fulminant forms of hepatitis, while genotype B associated with liver cirrhosis [9-11].
This study aimed to determine the prevalence of occult HBV and HBV genotypes among renal transplant patients in Khartoum State, Sudan.
During the time frame of the present study (2015), 100 renal transplant patients were recruited from Dr. Salma and Ibin-Sina Hospital centers for transplantation and hemodialysis, Khartoum, Sudan, Blood samples were collected in EDTA container and centrifuged at 3000 rpm for 5 minutes. Obtained plasma samples were then labeled and stored at -20°C until further analyses.
Commercial ELISA kits including sandwich ELISA for HBsAg (Cut-off =(mean NC × 2.1; OD=450 nm; positivity of >1.0) and competitive ELISA for anti-HBc (Cut-off=(mean NC × 0.5; OD = 450 nm; positivity of <1.0) (Diagnostic Automation/Cortez Diagnostic Inc. USA) were used according to the procedures described by the manufacturers. All samples with negative HBsAg and positive results for HBc antibodies were further processed for DNA extraction.
DNA was extracted from patient’s plasma materials using commercial Kit (Analytik Jena, Germany) according to manufacturer’s instructions. Briefly, 200 µl of lysis solution CBV/Carrier Mix into a 2.0 ml reaction was added to 200 µl plasma sample and 20 µl of proteinase K, and then mix and incubated at 70°C for 10 minutes. Subsequently, 400 µl of binding solution SBS was added to the sample after which all the lysis mix of the resulting solution was applied to a column. A volume of 500 µl of washing solution HS and 650 µl washing solution LS two time was added for washing and the nucleic acids were eluted with 60 µl pre- heat RNase-free water and stored at -20°C until used.
The PCR was performed by processing the extracted DNA from plasma with primers that are specific for the HBsAg gene of HBV. The primers used consisted of a forward primer
5’-TCGGAAATACACCTCCTTTCCATGG3’ HBV genome (353- 1377) and reverse primer, 3’GCCTCAAGGTCGGTCGTTGACA-5’ HBV genome (702-1681), optimized PCR reaction for HBV amplification was performed according to Mohammed AA, et al. [12]. 10 µl of the amplified product was subjected to direct analysis by gel electrophoresis in 2% agarose. The product was visualized by using UV solo TS Gel documentation system, (Analytik Jena, Germany). The expected size of surface antigen gene amplicon was 350 bp.
A genotyping system based on a two-tube Multiplex Nested PCR method? Using type-specific primers was used in assigning Hepatitis B genotypes A-F based on pre-S1 through S genes. The HBV primers S1-2 and P1 were universal outer primers. Primer B2 was used as the inner sense primer with a combination of other antisense primers for HBV genotypes A, B, and C in a multiplexing system named “Mix A”. Primer B2R was used as the antisense inner primer with a combination of sense primers for HBV genotypes D, E and F in a multiplexing system named “Mix B”. The HBV genotype specific primers used have been designed based on the conserved nature of those sequences within a genotype and optimized for Multiplex Nested PCR reaction for HBV amplification according to [13,9]. The system of mix A and B have passed on only one program containing different parameters. The samples were visualized on a gel documentation System. The expected band size of a genotype of Mix A is Type A-68bp, type B-281 bp and type C-122 bp and Mix B type D-119 bp, type E-167 bp, and type F-97 bp [9].
The Real-time PCR was performed by using Hepatitis B Viral DNA Quantitative Fluorescence Diagnostic Kit (fast HBV, PCR-Fluorescence Probing) (Sansure-Biotech Inc. China) according to manufacturer’s instructions. Briefly, 5 µl of each plasma specimen, positive control, and negative control, quantitative reference A, B, C, D standards were transferred directly to a PCR reaction tube and thoroughly mixed it with 5 µl DNA lysis buffer and then incubated for 10 minutes.
In another PCR tube PCR-master mix was prepared using 38 µl of HBV PCR mix, 2 µl of enzyme mix and 0.2 µl internal controls. The lysat was then mixed with PCR master mix. The final volume was 50 µl for a single reaction. The thermal cycling conditions were 2 minutes at 50°C, 2 minutes at 94°C for initial denaturation and 45 cycles of 15 seconds at 94°C for denaturation and 30 seconds at 57°C for annealing and extension and finally cooling for 10 seconds at 25°C. The reaction was performed using Rotor-gene Q Real time PCR machine (Qiagen, Germany).
All data were statistically analyzed by statistical package of social science (IBM SPSS version 20.0) for windows software package. A probability ≤ 0.05 was considered statistically significant result. Kappavaue was evaluated as follows, values<0 no agreement; 0-0.20 slight; 0.21-0.40 fair; 0.41-0.60 moderate; 0.61-0.80 substantial and 0.81-1 almost perfect agreement.
A total of 100 samples were tested for HBsAg, Ninety-seven samples (97%) were negative for HBsAg by ELISA. The 97 samples (that were HBsAg negative) were then tested for anti-HBc using ELISA. Out of these, 60 samples (61.9%) were negative for anti-HBc, while thirtyseven samples (38.1%) samples tested positive for anti-HBc (Table 1). According to the gender, anti-HBc was detected in 23(62.2%) males and 14(37.8%) females (Table 1).
| Gender | Anti-HBc | Total | |
| Positive | Negative | ||
| Male | 23(62.2%)(42%)* | 32(53.3%)(58%)* | 55(56.7%)100% |
| Female | 14(37.8%)(33%)** | 28(46.7%)(67%)** | 42(43.3%)100% |
| Total | 37(38.1%)*** | 60(61.9%)*** | 97(100%) |
Table 1: Frequency of Anti-HBc according to gender.
* % out of total males; ** % out of total females; *** % out of total HBsAg negative patents.
A total of 37 samples that were negative for HBsAg and positive for anti-HBc were tested for HBV DNA using PCR, Real time PCR and for HBV genotypes using Multiplex Nested PCR. HBV DNA was detected in 0(0.0%) samples using PCR and in 19(51.4%) samples using Real time PCR, while different HBV genotypes (A, D and E) either singly or mixedly were detected in 24(64.9%) samples using Multiplex Nested PCR. According to gender, DNA was detected by Multiplex Nested PCR in 15(65.2%) males and 9(64.3%) females, and by using Real time PCR in 11(47.8%) males and 8(57.1%) females (Tables 2 and 3). The genotypes of HBV and their numbers detected using Multiplex Nested PCR were as follows; A-0(0%), D-0(0%), E-9(37.5%), D/E-13(54.1%), A/D-1(4.2%) and A/D/E-1(4.2%). The frequency of Occult DNA was highest in the above 40 years old age group and no occult DNA was detected in the under 15 years old age group (Table 4) (Figures 1-3).

Figure 1: Agarose gel result for HBV genotypes using Multiplex nested PCR (lane 8: 100 bp ladder; lane 1: Negative control; lane 2, 3, 4, 5, 6, 7: Mixed genotypes D and E).
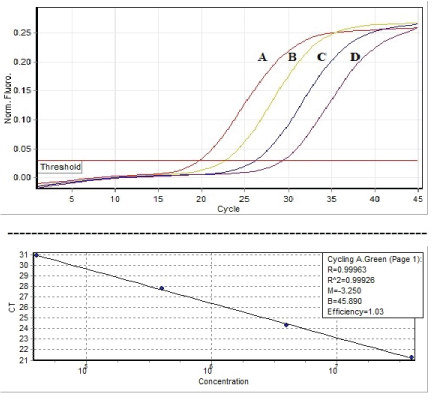
Figure 2: Graphic depiction of the linear range of quantitative references A, B, C, D standard curve detection (104 , 105 , 106 , 107 IU/ml) of Hepatitis B virus by Sansure kit, Fast HBV Real Time PCR assay.
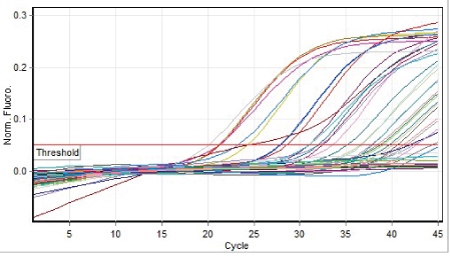
Figure 3: Graphic depiction of the linear range of positive control, negative control, quantitative references and positive samples, using Hepatitis B virus by Sansure kit, Hepatitis B Viral DNA Quantitative Fluorescence Diagnostic Kit.
| Gender | Total tested | PCR | Multiplex Nested(c)PCR | Real time PCR(d) |
| Male(a) | 23 | 0(0%) | 15(65.2%) | 11(47.8%) |
| Female(b) | 14 | 0(0%) | 9(64.3%) | 8(57.1%) |
| Total | 37 | 0(0%) | 24(64.9%) | 19(51.4%) |
Table 2: Frequency of Occult HBV in HBc positive patients using PCR,
Multiplex Nested PCR and Real time PCR according to gender.
(a) Real time PCR-Multiplex Nested PCR (P<0.05); (b) Real time PCRMultiplex Nested PCR (p=0.015)
(c) Multiplex Nested PCR (p=0.47); (d) Real time PCR (P<0.05)
| Samples/Test | PCR | Multiplex Nested PCR | Real time PCR |
| Positive | 0(0%) | 24(64.9%) | 19(51.4%) |
| Negative | 37(100%) | 13(35.1%) | 18(48.6%) |
| Total | 37(100%) | ||
Table 3: Overall frequency of Occult HBV using PCR, Multiplex Nested PCR and Real time PCR among 37 patients enrolled in the study.
| Age group in years | Number of patients with HBV viruses (%) | |||
| Total tested | PCR | Multiplex Nested PCR | Real time PCR | |
| ≤ 15 | 6(16.2%) | 0(0%) | 0(0%) | 0(0%) |
| 15-40 | 11(29.7%) | 0(0%) | 9(81.8%) | 7(63.3%) |
| ≥ 40 | 20(54.1%) | 0(0%) | 15(75%) | 12(60%) |
| Total | 37(100%) | 0(0%) | 24(64.9%) | 19(51.4%) |
Table 4: Frequency of Occult HBV using PCR, Multiplex Nested PCR and Real time PCR according to age group.
| PCR | Multiplex Nested PCR(a) | Real time PCR(b) | ||
| Positive | Negative | Positive | Negative | |
| Positive | 0 | 24 | 0 | 19 |
| Negative | 0 | 13 | 0 | 18 |
| Total | 0 | 37 | 0 | 37 |
Table 5: Cross tabulation of Multiplex Nested PCR and Real Time PCR
results vs. PCR results.
(a),(b) Kappa value=0.000
| PCR |
|
Total | ||||
| Positive | Negative | |||||
| Multiplex Nested PCR | Positive | 15 | 9 | 24 | ||
| Negative | 4 | 9 | 13 | |||
| Total | 19 | 18 | 37 | |||
Table 6: Cross tabulation of Real time PCR and Multiplex Nested PCR results.
Using Multiplex Nested PCR the prevalence was not significantly higher (P.value=0.47) in males than female, like while, using Real time PCR the prevalence was not significantly higher (P.value=1) in males than in females. When comparing Real time PCR and Multiplex Nested PCR, no significantly more females (P.value=1) were detected by Multiplex Nested PCR than Real time PCR, however significantly more males (P.value=0.015) were detected by Multiplex Nested PCR than Real time PCR. Kappa values for Real time PCR and Multiplex Nested PCR vs. PCR was 0.0 indicating no agreement, but fair agreement (Kappa value was 0.292) was found between Multiplex Nested PCR and Real time PCR (Tables 5 and 6).
The present study was aimed to determine occult hepatitis B and hepatitis B genotypes among renal transplant patients in Khartoum, Sudan. Ninety-seven (97%) of our study subjects were negative for HBsAg, of whom 37(38.1%) were positive for HBc antibodies. Similar result were reported in Venezuela, India, and Iran by Gutierrez C, et al., Duseja A, et al., and Sofian M, et al. respectively [14-16], but these authors were not able to detect occult hepatitis B in their tested subjects. However, Mohammed AA, et al., [12] were able; using the same primers as ours; to detect occult HBV in 3(3.3%) of haemodialysis patients in Khartoum State, Sudan. In the present study, we were able to detect OBI in 24.7% and 19.6% of renal transplant patients by using Multiplex Nested PCR and Real time PCR respectively.
The failure of PCR to detect occult HBV in our study population is unclear, but this may indicate a higher sensitivity of Multiplex Nested PCR and Real time PCR to detect occult hepatitis B infections. The occult HBV prevalence in the study as determined by Multiplex Nested PCR for genotypes (24.7%) and Real time PCR (19.6%) is similar to the prevalence in haemodialysis, renal transplant patients and other groups that ranges between 0 and 58% reported from different countries such as Germany, Italy, Brazil, Mexico [17-20].
The high prevalence of OBI (51.4%) using Real time PCR and 64.9% using nested among HBc positive patients may be due to the fact that our patients were subjected to haemodialysis for various periods before kidney transplantation. This may have increased their risk of acquiring HBV and subsequently occult HBV infection.
The findings of the present study were higher than those reported by Peres AA, et al. [21], and by Bae E, et al. [22]; who found prevalences of 2% and 2.7%, respectively
There are no previous reports on the monitoring of occult HBV genotype using Multiplex Nested PCR among renal transplant patients where most of the studies were done using conventional PCR for Hepatitis B surface antigen gene or other hepatitis B genes, this may indicate that the conventional PCR methods for detecting OHB in renal transplant patients need to be reassessed.
In the present study, 3 different genotypes (A, D and E) were detected. Singular infections were only found with genotype E while mixed infections with 2 or 3 genotypes represented the majority of cases. This may be related to mutations, genetic variations or other causes. The genotypes E-9(37.5%), D/E-13(54.1%) were the dominant infections in our study which is in contrast to a study from Egypt by Maysaa ESZ, et al. [23] carried out in hemodialysis patients that reported genotypes C(44.4%), A(27.8%) and B(22.2%) as the dominant genotypes.
Hepatitis B virus genotyping using Multiplex Nested PCR is simple, easy and cheaper than sequencing but sequencing and phylogenetic analysis based on nucleotide sequences produces the most accurate genotyping results. However, phylogenetic analysis is not an appropriate method for diagnostic testing in laboratories with limited resources and is more suitable for developing countries where advanced molecular testing is available. Real time PCR, on the other hand, could be more suitable method for testing for occult hepatitis B virus in developing countries but as indicated herein may miss some of the infections.
Occult HBV showed moderate prevalence in renal transplant patients in Khartoum State and can only be detected by using Multiplex Nested PCR and Real time PCR. In addition, our study may indicate that Occult HBV detection may require the use of more than one technique.
The study was approved by the Ethical Review Committee (ERC) of Central Laboratory, Ministry of Higher Education and Scientific Research, Khartoum State, Sudan. Informed consents were obtained from adult patients or from parents or legal guardians of children.
Not applicable.
Data sharing not applicable to this article as no datasets were analyzed during the current study.
Authors have no competing interests to declare.
The Central Laboratory, Ministry of Higher Education and Scientific Research supported this work for Hepatitis viruses (2016HB10002-10, 2016HB10002-12).
We thank Ibin-Sina Hospital and Dr. Salma Center for transplantation and hemodialysis for allowing us to collect plasma samples from patients. This work was funded by the Central Laboratory, The Ministry of Higher Education and Scientific Research and Technology, Khartoum, Sudan.
MOM did the sample collection, ELISA, Multiplex Nested PCR, Real time PCR and drafted the manuscript. KME helped in the analysis of data and worked in the preparation and edition of the manuscript. IME contributed to the conception and design of the study and helped in the drafting of the manuscript. AME contributed to the conception and design of the study, the drafting of the manuscript. All authors read and approved the final manuscript.
Download Provisional PDF Here
Aritcle Type: RESEARCH ARTICLE
Citation: Mustafa MO, Enan KA, Elkhidir IM, El Hussein ARM (2019) Occult Hepatitis B Virus and Hepatitis B Genotypes among Renal Transplant Patients in Khartoum State, Sudan. J Emerg Dis Virol 5(1): dx.doi.org/10.16966/2473-1846.147
Copyright: © 2019 Mustafa MO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access