Abstract
Leptospirosis is a globally re-emerging neglected zoonotic disease that continues to be a significant human and veterinary public health
concerns, with 0.1-1/100,000 population and estimated 350,000-500,000 severe cases annually (International Leptospirosis Society surveys). The
disease is caused by infections of pathogenic spirochetes of the genus Leptospira which are classified into 20 genomospecies and placed more
than 250 serovars/strains. Treatment by antibiotics such as doxycycline, ceftriaxone, azithomycin is predominant. Antibiotics provide therapeutic
activity when initiated early of illness, and might be less effective at late and severe of human leptospirosis such as Weil’s disease and severe
pulmonary hemorrhagic syndrome and also in animal reservoirs. Besides, antibiotics might cause adverse effects, i.e., Jarisch-Herxheimer reaction,
due to massive release of the bacterial toxic substances. An immunomodulation or passive immunotherapy by using therapeutic antibodies against
the Leptospira virulent factors might be the optimal therapeutic approach for the late and severe leptospirosis. LipL32, an immunodominant
outer membrane protein of pathogenic Leptospira spp. has been used as diagnostic biomarker, vaccine candidate for a broad spectrum vaccine
development, and therapeutic target for passive immunotherapy of leptospirosis. Passive immunotherapy of experimental leptospirosis by using
therapeutic mouse monoclonal antibodies and their single-chain variable fragment antibodies specific to LipL32 have been demonstrated.
Keywords
Leptospirosis; Re-emerging zoonosis; Passive immunotherapy; Therapeutic monoclonal antibody; LipL32
Leptospirosis remains one of the most common zooanthroponotic
infections found throughout the world, particularly in subtropical and
tropical areas, such as central and tropical areas of America, Oceania, and
Southeast Asia [1-3]. In Southeast Asian countries, the disease is endemic
in Thailand, Lao PDR, Philippines, Indonesia, Malaysia, and Vietnam [2-
7]. For industrialized countries, leptospirosis cases tend to be imported
subsequent to travel to the disease endemic areas [4-6]. World health
organization has estimated incidence of leptospirosis is 0.1-1 case per
100,000 populations annually. The incidence of leptospirosis in Thailand
is 10 per 100,000 populations with a 19.7% fatality rate in 2011-2012 [8,9].
The disease is caused by infections of pathogenic spirochetes in the
genus Leptospira. Within the genus, more than 250 serovars/strains of
the spirochetes are classified into 20 genomospecies based on 16S rRNA
relatedness [2]. According to pathogenicity in human, Leptospira spp. are
placed into three clades: pathogenic (cause disease), non-pathogenic (freeliving
saprophytic, do not infect), and intermediate (infect and may cause
disease). Pathogenic Leptospira spp. infects both humans and animals
such as ruminants, i.e., cattle, buffalo, sheep, goat, horse, and swine,
domestic animals, rodents, and wildlife. Animal infections may lead to
a variety of clinical outcomes including jaundice, infertility, abortion in
the late pregnancy, still-birth, failure to thrive, decreased milk production,
and death. Leptospira spp. are highly adapted to a broad range species,
including environments, mammalian reservoirs to human [10]. Animal
reservoirs shed Leptospira in urine to soil, water and environments and
the spirochete infects human at skin and mucosae such as conjunctival,
oral, and genital surfaces [11,12]. Risk associated leptospirosis are
occupational health and safety regulations such as veterinarians, abattoir
workers, animal caretakers, gardeners or farmers [1,10]. In recent years,
outbreak of human leptospirosis is found in fresh water sports, such as
caving, canoeing, kayaking, rafting, and triathlons. The disease is also a
travel-associated bacterial zoonosis [6].
Clinical symptoms of leptospirosis ranges from asymptomatic,
non-specific and self limiting febrile illness including chill, headache,
fever, which may be confused with other acute febrile illness such as
influenza, malaria and dengue fever to life-threatening illness. Muscle
pain, myalgias, and ocular suffusion are also present. Weil’s disease,
severe pulmonary hemorrhagic syndrome and meningoencephalitis
are severe forms of leptospirosis which characterized by renal, hepatic,
cardiac and neurologic complications leading to jaundice, acute renal
failure, hemorrhage, meningitis and septic shock, and multi-organ failure.
Leptospirosis-associated severe pulmonary hemorrhagic syndrome has
been reported sporadically or in the disease outbreaks with 5-40% fatality
rate [13,14]. Antibiotics such as doxycycline, ceftriaxone, azithromycin,
penicillin G, ampicillin have been used for treatment of leptospirosis.
Doxycycline has been used in pre- or post-exposure prophylaxis.
Severe leptospirosis requires both antibiotics and supportive therapy for
improving mortality rate. Treatment with antibiotics may prevent disease
severity from mild to severe forms if antimicrobial therapy initiated early
and preferably before the fifth day after the onset of the illness. For mild
symptom, beta-lactam class antibiotics such as penicillin and amoxicillin
are useful. Third-generation cephalosporin in treatment of leptospirosis
is exists [15,16]. Aminoglycodises have been considered in the past [17].
Most of the antibiotics treatment may cause adverse effects, i.e., JarischHerxheimer
reaction (JHR), a ‘cytokine storm’ caused by the massive
release of bacterial toxic substances after administrating antibiotics
[18,19]. The JHR associated leptospirosis was reported to be 80% in a
Malayan study. Doxycycline-associated phototoxicity and gastrointestinal
side effects have been reported.
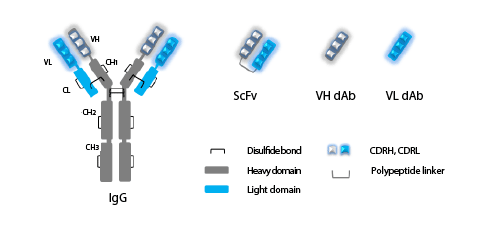
Figure 1: Immunoglobulin G (IgG), single-chain variable fragment (ScFv) and single-variable domain (dAb) antibodies.
IgG antibody consists of two identical heavy chains (grey) and two identical light chains (blue). Heavy chain consists of one variable domain (VH) and
three constant CH1, CH2, and CH3 domains, Light chain consists of one variable (VL) and one constant (CL) domain. Complementarity determining
regions (CDRH, CDRL) in variable domains are indicated. Full-length IgG contains two antigen-binding regions responsible for binding to specific
antigen and constant Fc region (CH2, CH3) responsible for interaction with Fc receptors. Single-chain variable fragment antibody (ScFv) consists of
variable domains of heavy (VH) and light (VL) chains joined by a peptide linker.
Outer membrane of pathogenic Leptospira displays various
components play roles in bacterial pathogenesis by acting as adhesins,
porins and receptors. LipL32 is regarded as a dominant lipoprotein
located at the outer membrane. The LipL32 protein is restricted to
pathogenic and intermediate clades of Leptospira serovars. Sequence
homology of LipL32 of pathogenic serovars showed more than 94% and
decreased to 67% homology in intermediate clade of Leptospira spp.
[20-22]. Pathogenic Leptospira expresses LipL32 constitutively in both
in vitro culture and during infection in mammalian hosts [20,23]. The
protein is highly immunogenic, i.e., LipL32-specific IgG can be detected
in acute and convalescing leptospirosis patient’s sera [21,23-24]. Thus,
LipL32 is a molecular target for leptospirosis diagnosis [24-26]. It is also
a potential immunogen for developing universal leptospirosis vaccines
[27-29]. LipL32 exhibits hemolytic activity and enhance the hemolytic
activity of sphingomyelinase-H (SphH), hence its synonym, hemolysisassociated
protein-1(Hap-1) [30-31]. LipL32 was also identified as a
member of the Leptospira adhesive matrices (MSCRAMMs), responsible
for binding to extracellular matrix (ECM) molecules, including matrigel,
laminin, collagens (I and IV) and both intact to 30 and 45-kDa proteolytic
fragments of fibronectin (FN) [32-33]. LipL32 also binds to the zymogen
plasminogen to generate plasmin [34] which adheres to the proteoglycan
of human cell surface receptors [35], to cultured mammalian cells [36] and
to neutrophils [37]. Passive immunotherapy of experimental leptospirosis
by using LipL32-specific monoclonal antibodies (mAbs) and recombinant
antibody fragments have been demonstrated. [38-39]. Thus, LipL32, an
immunodominant outer membrane protein of pathogenic Leptospira spp.
has been used as diagnostic biomarker, vaccine candidate for a broad
spectrum vaccine, and therapeutic target in passive immunotherapy for
leptospirosis.
Passive immunotherapy by using therapeutic monoclonal antibodies,
conjugated antibodies, bispecific antibodies, antibody fragments such as
Fab, F(ab’)2, single-chain variable fragment (ScFv), single-variable (dAb)
domain have been developed and used as non-drug therapeutic agents for
treatment and intervention of infectious diseases, cancer, inflammatory
and autoimmune diseases, intoxications, and envenomations [40-42].
Antibody therapy of experimental leptospirosis by monoclonal antibodies
(mAbs) directed against agglutinating serovar-specific lipopolysaccharide
have been demonstrated in animal model [43-45]. Two murine
hybridoma clones secreting monoclonal antibodies, namely mAbLPF1
and mAbLPF2, specific to the Leptospira LipL32 outer membrane protein
have been produced [37]. Both mAbs neutralized Leptospira-mediated
hemolysis in vitro, and exhibited therapeutic activity when passively given
to experimental hamsters infected with Leptospira spp. [37].
Single-chain variable fragment antibody (ScFv; VH-linker-VL)
molecule [42] is an effective therapeutic small molecule with an expected
lower (or lack of) immunogenicity and better target epitope accessibility.
The molecular mass of ScFv is about 30 kDa compared to the 150 kDa of
intact IgG lacks of functional domain (Fc) of immunoglobulin. Murine
single chain antibody fragments, as well as humanized-ScFv have been
produced from the original mouse mAbLPF1. The scFv exhibited
therapeutic activity when passively given to experimentally hamsters
infected with heterologous Leptospira [39]. Therapeutic LipL32 epitopes
and membrane binding inhibitory activity of mAb to MDCK monolayer
cells were also investigated [36]. The epitope peptide of mAb LPF1 was
mapped to a non-contiguous carboxy-terminal β-turn and amphipathic
α-helix of LipL32 structure contributing to phospholipid/host cell adhesion
and membrane insertion. We found that the mAbLPF2 epitope was located
on the interacting loop of peptide binding groove of the LipL32 molecule
responsible for interactions with host constituents. Epitope sequences are
highly conserved among Leptospira spp. and are absent from the LipL32
super family of other microorganisms. Both epitopes are surface-exposed,
readily accessible by mAbs, and immunogenic. However, they are less
dominant when revealed by LipL32-specific immunoglobulins [36].
Therapeutic antibodies, particularly the humanized-ScFv, have potential
for further development as a non-drug therapeutic agent for human
leptospirosis, especially in subjects allergic to antibiotics.
Acknowledgement
Scholarship for academic research presentations aboard (2015) was
supported from ICTM grant, Faculty of Tropical Medicine, Mahidol
University and from Faculty of Graduate Studies, Mahidol University.
Article Information
Article Type: Short Communication
Citation: Maneewatchararangsri S (2016)
Therapeutic Monoclonal Antibodies and Their
Engineered Antibody Fragments Specific to LipL32
for Passive Immunotherapy of Leptospirosis. J Emerg Dis Virol 2(2): doi http://dx.doi.org/10.16966/
2473-1846.114
Copyright: © 2016 Maneewatchararangsri S. This
is an open-access article distributed under the
terms of the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 28 Jan 2016
Accepted date: 11
Feb 2016
Published date:19 Feb 2016