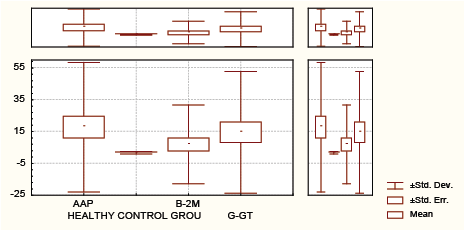
Graph 1: Distribution of alanine aminopeptiDase (aap) , γ-glutamil transferase (γ−GT),β2M(urine).
Spasovski Dejan*
University Clinic of Rheumatology, “Ss Cyril and Methodius” University, Skopje, Republic of Macedonia*Corresponding author: Spasovski Dejan, University Clinic of Rheumatology, “Ss Cyril and Methodius” University, Skopje, Republic of Macedonia, Tel: +389 2 3147-668; E-mail: drspasovski@yahoo.co.uk
Aim: To compare diagnostic values and laboratory variables of alanine - aminopeptidase (microsomale AAP), γ-glutamyl transferase (γ-GT), β2-microglobuline (β2-M), C Reactive Protein (CRP) and index for disease activity (PASI) in early diagnosis in previously untreated Psoriatic arthritis (Psa). To determine the effect of untreated Psoriatic arthritis on tubular function, sensitivity of the Brush Border region as well as the diagnostic value of the enzymes originating from proximal renal tubules.
Methods: From the standard methods of the International Federation for Clinical Chemistry (IFCC) we used the kinetic method for determination of alanine - aminopeptidase (microsomal AAP), γ-glutamyl transferase (γ-GT) and MEIA (Microparticles Enzyme Immunoassay(Abbot Ax sym system) for determination of β2-microglobuline in urine. We examined samples (serum and urine) from 70 participants (35 Psa untreated, 35 healthy control group). RF and CRP are determined with Latex agglutination test in the same participants.
Results: From 35 examined patients with Psa, 12 pts showed presence of AAP enzymuria (test sensitivity was 34.28%), 8 pts showed presence of γ-GT (test sensitivity was 22.85%), while the presence of β2-microglobuline in urine was low (test sensitivity 0%).
Conclusions: AAP has better sensitivity than γ-GT and β2-microglobuline in the detection of asymptomatic renal endothelial changes in untreated Psa.
Alanine-Aminopeptidase (AAP); γ-Glutamyl Transferase (γ-GT); β2-Microglobuline (β2-M); Psoriatic Arthritis (Psa)
Brush border region (brush, layer, striated) is composed of microvilli covered with simple cubic and cylindrical epithelium, found in different location of the body. Diameter of the microvilli is 100 nm, while their length vary from 100 nm to 200 nm. Because microvilli are so small and dense in the brush border epithelium, they could be seen only with electronic microscope. With light microscope they could be usually seen collectively as ‘fuzzy fringe’ (feathered, fibrillary, edgy, borderline), aspartof the surfice of the epithelium. The appearance of the ‘fuzzy fringe’ determines the name Brush border, because this structure resembles the painter brush.
Brush epithelial cells are found in two main locations in the human body.
It is the place where absorption takes place. Brush epithelium in the intestinal cover layer is the place of terminal carbochydrate digestion. Microvilli that compose brush epithelium contain enzymes for this terminal part of digestion, located in the apical plasmatic membrane as an integral membrane proteins. These enzymes are located close to the transpoters, which enables absorption of the digested food.
In the kidneys where the brush epithelium is useful to make difference between proximal tubuls (that posses brush epithelim) and distal tubules (that do not posses).
Brush border morphology with the brush epithelium, increases the cell surface, especially useful for absorption. The cells that absorb substances have great necessity of contact surface with substances in order to be efficaceous. The luminal surface of the epithelial cells from this segment of the nephron is covered with densly packed microvilli that form border, whish can be seen under light microscope. The microvilli in great measure increase the luminal surface of the cells which in great measure facilitate their resorptive function. Membranes inverted inside that form microvilli are the places for numerous sodium pumps.
The cell cytoplasm is densly filled with mitochondria, found mostly in the basal region, inside the curves of the basal plasmatic membrane. The high quantity of the mitochondria gives the cells acidophilic appearance. The mitochondria are necessary for energy supply for the active transportation of the sodium ions outside the proximal tubules. The water passively follows sodium outside the cells according to the concentration gradient. The cubic epithelial cells that cover proximal tubules have extensive lateral interdigitationes between adjasent cells, that seems as there are no cell borders seen under the light microscope. The end resorption of the content of the proximal tubules after drugintake or stop in circulation (in capilars) around tubules leads to disturbance of the cell morphology of the proximal tubule cells, including ejection of the nucleus in the tubular lumen, giving it dirty look in contrast to the clear appearance of the distal tubules, that have completely different caracteristics.
Microvilli have the characteristics of PH parturition. It is the tendency of the acid matters to accumulate in the alkaline fluid compartments, while alkaline matters in acid compartments. So, acid drugs are secreted in great quantities when the urine is alkaline, and vice versa, alkaline drugs are secreted in great quantities when the urine is acid.
The aim of this study is to determine the effect of untreated Psoriatic arthritis on tubular function and sensitivity of the Brush Border of the proximal tubules. AAP, γ-GT, β-2M are used as indicators for proximal tubular damage.
Several classes of measurable proteins in urine are used for evaluation of the renal disfunction.
Alanine–aminopeptidase (AAP), (aryl amide amino acid, aminopeptidase, α-amino-acyl-peptide-hydrolase (microsomal) AAP, ES 3.4.11.2, previously 3.4.1.2) is a hydrolytic derivative of peptides, amides and p-nitroanilide.
AAP is found in numerous tissues, mostly in kidney, intestine, lungs and liver. AAP in different tissues has different electrophoretic conductivity. This enzyme has at least five different isoenzymes that could be separated from each other with electrophoresis, ion-changing chromatography or immunologically. In normal serum is found only one isoenzyme, while in hepatobilliar or pancreatic diseases are found additional fractions. The enzyme is also found in urine.
γ-glutamyl transferase (γ−GT),(γ-glutamyl-peptide amino acidγ- glutamyl transferase, ES 2.3.2.2.γ−GT catalyzes the transfer of (γ-glutamyl groups with peptides (as glutathione) to other peptides or amino-acids.
γ−GT plays an important role in the glutathione metabolism. High enzyme concentrations are found in kidneys (proximal tubule), pancreas (acinar cells), prostate and liver. γ−GT is mostly located on the external part of the plasma membrane [7]. γ−GT isoenzymes in serum are result of the different post-translational modifications as for example complex formations with lipoproteins or modifications of the carbohydrate part of the γ−GT molecule [8]. The possibility of the presence of isoenzymes in different tissues (liver, pancreas, kidney, and duodenum) is due to the differences in carbohydrate part of the γ−GT molecule. Although the peptide part of the enzyme molecule is the same no matter the tissue of origin, these isoenzymes differ in kinetic, electrophoretic and immunological features.
The tubular function could be evaluated with mesurements of the excreted low molecular proteins in urine. β2-microglobuline (β2-M) is used as an indicator of the tubular disfunctions in glomerulonephritis [9] and is often used as sensitive marker for evaluation of the renal function [10-12]. β2-M is a small polypeptyde with low molecular weight (11.815 daltons). β2-M contains light chains of the main histocompatibility antigen (HLA). It influences production of the RF (IgM class). In normal individuals, β2-microglobuline is found both in serum and urine. 95% of the free β2-M is ultrafiltrated through renal glomerules and almost completely is reapsorbed in 99.9% with proximal tubular endocitosis and finally is catabolized in amino acids in healhty individuals. Due to this mechanism, normally in urine are detected in traces. Imparement in the glomerular filtration leads to increase in serum β2-M, while tubular damage leads to rise in urine β2-M.
Serum concentration of β2-M depends on the glomerular filtration rate (GFR) and shows significant negative correlation with inulin clearence. These findings show that with determination of the serum level of β2-M one could get an index for disfunction of the renal gromerulus.
In some pathological conditions, increased quantities of β2-M are excreted in urine. It happens when β2-M serum concentration exceeds the renal threshold. The serum level of β2-M depends on the ratio of synthesis and release in serum pool and its relation with clearence. Such conditions are notified in patients with inflammatory diseases (Rheumatoid arthritis, SLE, Sy.Sjögren, Crohn disease, cancer, liver damage). β2-M concentration in urine could be increased also when reapsorption is decreased due to renal proximal tubular damage. Proximal tubular disfunction results with elevated concentrations of urine β2-M, alowing to make distinction between proximal tubular from glomerular renal impairment.
β2-M is used for evaluation of the GFR and renal tubular function, especially for tubulotoxic effect of different substances, such as heavy metals (cadmium and lead) and as a screening test for early detection of Balcan nephritis in regions where it is edemic. In urine β2-M is unstable if ph<6 and it is recommened to alkalize the urine with bicarbonates before it is tested. β2-M is considered the earliest protein of tubular proteinuria.
Diagnosis of the patients included in the study is based upon revised diagnostic criteria for Classification of Psoriatic arthritis from 2005, proposed by the American Association for Rheumatic arthritis (ARA) [13]. Clinical evaluation for disease activity and disease diagnosis is based upon diagnostic criteria of Moll-Wright for Classification of Psoriatic arthritis [14]. Patients are dermatologically tested, including examination of the psoriatic changes of nails, psoriatic areas and disease activity index (PASI) as well as evaluation of the peripheral and axial joints [15]. Oligoarthritis is taken in consideration when <5 joints are involved and polyarthritis when ≥ 5 joints are involved. Symmetric arthritis is considered when bilateral joints are involved >50%.
In the study are included 35 patients (8 women, 27 men) suffering from PsA and 35 patients (23 women, 13 men) as healthy control group. Median age was 50.18 years (SD ± 8.09) (35-65 years) in PsA group, while 48.2 years (SD ± 10.19) (29-65 years) in healthy control group. Median disease duration was 30.17 (SD ± 40.13) in the interval of 1-60 months. None of the patients included in the study has previous or current history of renal disese. None of them previously used NSAIDs. The others negate use of other drugs before entering the study, especially drugs from the base line therapy such as methotrexate, antibiotics or diuretics. Samples are collected in the period of two years.
In the study are included patients suffering from Psoriatic arthritis, aged 18-65 years old, newly diagnosed and previously untreated.
From the study are excluded all the patients with diseases or conditiones that can directly or indirectly influence the results, such as:
All the patients took place in the study voluntarily, so the ethic criteria for inclusion in the study are fulfilled.
For clinical evaluation of the disease one have to examine the following parameters: complete blood count (CBC) and differential, reactants of the acute phase such as C-Reactive Protein (CRP), Rheumatoid factor (RF), Erythroid Sedimentation Rate (ESR), Aspartate-Aminotransferase (AST), Alanine-Aminotransferase (ALT), Creatinine Kinase (CK), Lactate– Dehydrogenase (LDH), serum urea and serum creatinine. The urine samples were taken not only for routine analyses, but also to determine the levels of AAP, γ−GT, β−2M. Due to the urine instability of β−2M <6 pH it is recommended the urine to be alkalized before testing.
Serum creatinine is determined according to “Jaffe” method. Referent values are: serum creatinine 45-109 µmol/L, urine creatinine 7-17 µmol/L.
CRP is determined with the agglutination test (Latex CRP test) (BioSystems S.A. Reagens & Instruments Costa Brava 30, Barselona, Spain). Referent values are: serum CRP<6 mg/L.
RF is determined with the agglutination test (Latex CRP test) (BioSystems S.A. Reagens & Instruments Costa Brava 30, Barselona, Spain). Referent values are: serum RF<8 IU/ml.
ESR was determined with Westergren method. Normal values are: for men 7-8 mm, for women 11-16 mm.
GFR (Creatinine Clearance) was estimated by Cockcroft-Gault Equation.
Alanine amino–peptidase (aryl amid aminoacid, aminopeptidase, α-aminoacyl peptide hydrolase (microsomal), ANA, ES 3.4.11.2, former 3.4.1.2.) is hydrolized by peptides, amids and p-nitroanilide. During the process of hydrolization of peptides N –terminal amino acid is seceded (firstly anilide). The activity of AAP is determined by the methods similar to those for determination of leucine aminopeptidase. In this method is used L-alanine-4-nitroanilide as a substrate. The catalytic concentration of AAP is directly proportional to the absorption of p-nitroanilide is measured on 405nm. Refernt values: urine AAP 0.25-0.75 U/mmol creatinine.
γ-glutamyltransferase (γ-glutamyl) – peptide amino acid γ-glutamyltransferase ES 2.3.2.2.(γ-GT) catalyzes transfer of γ-glutamyl groups with peptides (such as glutathione) on other peptides or aminoacids. γ-GT influences the release of glutamyl rest as glutamine acid. With transpeptidation glytamyl rest coud be transfered again on a supstrat (for example from γ-glutamyl-naphtylamide results γ-glutamyl- γ-glutamyl-α-naphtylamide) or other suitable acceptor (aminoacid, di- or tri-peptide). The most suitable acceptor is glycylglycine.
Methods for measurements of the activity of this enzyme in serum use aromatic amids as supstrats (γ-glutamyl-anilide and γ-glutamylnaphtylamide). The superficial supstrate peptide analogue γ-glutamylp-nitroanilide is most frequently used. It is suitable for determination of the enzyme activity kinetically and colorimetrically. γ-glutamyl--pnitroanilide latter is supstituted by L-γ-glytamyl-3-carboxy-4-nitroanilide (glucan) due to its high solubility. Glyculglycine was used as a supstrate acceptor and buffer, due to its high catalytic activity. This method is standardized by the International Federation of Clinical Chemistry – IFCC) and is considered as referent method [16].
The IFCC method for meaurement of concentration of the catalytic activity of serum and urine γ-GT is based on the principles developed by Orlowski, Meiser and Szasz, and their modification by Persijin and Van der Slik. As a supstrate donor is used L-γ-glytamyl-3-carboxy-4-nitroanilide. In the IFCC method Tris (hydroxymethyl aminoethan is substituted with glycylglycyne, which acts as buffer and supstrate acceptor. Magnesium which earlier was used for maintenance of L-γ-glytamyl-3-carboxy-4- nitroanilide in the solution in IFCC method is omitted. This method is specific for determination of the activity of γ-GT [17-20].
Referent values: γ-GT (urine) 0.84-1.80 U/mmol creatinine.
Principles: Determination of ax sym β2-microglobuline is based on MEIA technology (Micro particles Enzyme Immunoassay) and enables quantitative determination of β2-microglobuline in serum, plasma and urine in patients with Rheumatoid arthritis and renal impairment.
The reaction is based on the inter-reaction of β2-M with anti-β2-M antibody, forming a mutual complex. This complex reacts with the Matrix cell and is bound to them. A conjugate of alkaline phosphatase is added, it bounds to the complex, forming sandwich complex. To this complex is added 4-Methylumbelliferyl Phosphate (4-MUP), reacting with alkaline phosphatase from the complex and a fluorescent product - Methylumbelliferon with light blue colour is made. From the degree of the optic fluorescence depends proportionaly the concentration of β2-M. It is determined automatically (Abbot Ax sym system).
Taking in consideration that β2-M is very sensitive to changes in urine pH i.e., very quickly is degraded in low pH levels. If pH <6.0 it is monitored, and if it is acid it should be alkalized.
Referent values: β2-microglobuline (urine)–0.02-0.19 mg/L.
For testing the significance of differences between two arithmetical means, i.e. proportions the Student-t-test is used to compare the mean parameters of certain numerical parameters between groups, as well as Willcoxon-matched test for independent samples. Sensitivity and predictivity for positive and negative test of the examined markers is determined with the test for sensitivity and specificity. P-value between 0.05 and 0.1 is considered statistically significant. Analysis of the data is performed with the statistical package Statistica 7.0.
From the 35 examined patients with Psa, 12 pts (34.28%) showed presence of APP enzymuria, 8 pts (22.85%) presence of γ-GT, while low percentage (0%) presence of β2-microglobuline in urine. RF was present in 0 pts (0%).
In the 35 pts with Psa, APP sensitivity was 34.28%, γ-GT sensitivity was 42.85%, β2-microglobuline was 0% and RF sensitivity was 0% (Table 1 and Graph 1).

Graph 1: Distribution of alanine aminopeptiDase (aap) , γ-glutamil transferase (γ−GT),β2M(urine).
|
Psa untreated group NO 35 |
Healthy control group NO 35 |
|
Positive/negative |
Positive/negative |
AAP+>0,75 |
12/23 |
1/34 |
γ-GT+>1,80 |
8/27 |
0/35 |
β2 M+>0,19 (mg/L) |
0/35 |
0/35 |
RF+30 ≥ IU/ml |
0/35 |
0/35 |
CRP+12 ≥ mg/L |
13/22 |
1/34 |
Table 1: AAP, γ-GT β2-microglobulin and other laboratory variables in Psa and healthy control group
For AAP, γ-GT, β2 microglobulin as well as other laboratory variables in Psa, sensitivity, specificity, predictive values for positive and negative test, as well as accuracy are shown on table 2.
|
AAP Psa No 35 |
γ−GTPsa |
β2M |
RFPsa No 35 |
CRPPsa No 35 |
Sensitivity % |
34,28 |
22,86 |
0 |
0 |
37.14 |
Specificity % |
75,56 |
100 |
100 |
100 |
97,14 |
Predictive value forpositive test % |
52,17 |
100 |
0 |
0 |
92,86 |
Predictive value for negative test % |
59,65 |
56,45 |
50 |
50 |
60,71 |
Accuracy % |
65,71 |
61,42 |
50 |
50 |
67,14 |
Table 2: Diagnostic performances of AAP, γ-GT, β2Μ and other laboratory variables in Psa
AAP has better diagnostic performances than γ-GT and β2M taking in consideration sensitivity and specificity (sensitivity 34.28% vs 22.86% vs 0%) and almost equal specificity (specificity 75.6% vs 100 % vs 100%) in detection of renal impairment in untreated Psa.
In the standard medical rheumatology the biggest emphasize is put on Rheumatoid arthritis as the most exposed disease, neglecting somehow the other diseases especially seronegative arthropathies, probably due to their lesser extent.
The explanation for the renal tubular enzymes is increased exfoliative turnover of the epithelial cells in Psa, which is adequately present also in proximal tubular epithelial cells.
Of all enzymes greatest emphasize is put on NAG as dominant lysosomal tubular enzyme. Traditional treatment of Psa and RA, includes non-steroid anti-inflammatory drugs (NSAIDs), disease modification drugs (DMARDs), steroids and immunosuppressive cytotoxic drugs. Methotrexate in low dose regime is the most frequently prescribed drug from DMARDs, while Ketoprofen (Niflamr , Ketonalr) and Paracetamol from NSAIDs.
Enzymes in urine could originate from plasma, glands from the urogenital tract, epithelial cells of the urinary tract, white blood cells, erythrocytes and kidneys. There are 40 different enzymes in the urine belonging to different groups: oxydo-reductases, transferases, hydrolases, lyases, while isomerases and ligases are not found in urine. Presence of so many enzymes in urine indicates the dominant role of the kidneys in their excretion.
The urine enzyme activity in urine normally is low and increases in renal tubular cell damage. Urinary enzymes especially NAG, AAP and AF are very sensitive indicators of renal parenchymal damage in comparison with functional measurements such as glomerular filtration rate and creatinine clearance. Relatively low sensitivity to GFR could be explained with great functional reserves of the kidneys and their great compensatory ability.
AAP sensitivity is greater in comparison with γ-GT and β2M (34.28% vs 22.85% vs 0%), with approximately equal specificity (75.6% vs 100 % vs 100%).
Statistical relation of disease duration (in months) and AAP and γ-GT and β2M enzymuria p=0.00 points out that untreated Psa damages the renal tissue as one of the visceral manifestations of the disease.
Untreated Psa primarily damages tubular Brush Border region and enzymes originating from it has greater sensitivity [21-29].
AAP has greater sensitivity than γ-GT and β2M in asymptomatic renal lesions in untreated Psa. AAP and γ-GT could be used in everyday clinical practice in diagnosis of early, asymptomatic renal lesions.
Download Provisional PDF Here
Article Type: Research Article
Citation: Dejan S (2017) Specific Developmental Profiles of Lysosomal or Brush Border Enzymuria as Yeast Overgrowth Citozomal Enzymes, and Some Histochemistry Aspect of Renal Urinary Tubulary Markers in Patients with Seronegative Osteo Arthropatia. Int J Vaccine Immunizat 3(1): doi http://dx.doi.org/10.16966/2470-9948.116
Copyright: © 2017 Dejan S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access