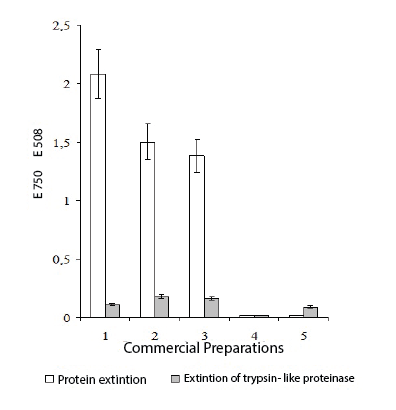
Figure 1: Activity of trypsin-like proteinase and protein extinction in the
commercial preparations received from donor blood.
Note: 1 - Immunoglobulin; 2 - Interferon; 3 – Herpetic vaccine; 4 -
Gonovaccine; 5 - Tularemic vaccine
V A Dіvocha*
Ukrainian Research Institute for Transport Medicine, Ministry of Health, Odessa, Ukraine*Corresponding author: V A Dіvocha, Ukrainian Research Institute for Transport Medicine, Ministry of Health, 65039 Odessa, Ukraine, E-mail: divocha09@ukr.net
The paper is devoted to study presence of components of host cell and its inhibitor in vaccines and blood preparations and to determine the presence trypsin-like proteinase and its inhibitor in vaccines and blood preparations. It is found that influenza virus vaccines (influvac, vaxigrip, fluarix), herpetic and tularemic vaccines contained inhibitor of trypsin-like proteinase in considerable quantity. Commercial preparations of human donor blood (immunoglobulin, interferon, fraxiparine and solcoseryl) contained both trypsin-like proteinase and its inhibitor. The immunoglobulin contained inhibitor 4,0 times more than interferon. Hence, the modern vaccines used for treatment are insufficiently purified and they should be used for treatment taking into account interaction of a flu virus with cellular components (enzymes and inhibitors).
Trypsin-like proteinase; Inhibitor; Vaccines; Immunobiological blood preparations
Our days, the vaccines play a leading role in flu and other virus infections prevention [1]. Periodical flu epidemics (1957 and 1959, 1968- 69, 1977-78, 2009-2010) covered up to 30% of the globe population within 9-10 months. According to the Ministry of Health of Ukraine, during an epidemic season 1998-99, 7% of the population had flu in Ukraine. In 2009-2010, during flu pandemic, than 5 million persons requested medical assistance, 1127 people died of which 100 pregnant women. The flu affected men, women and children of different age and nationalities. The analysis of epidemics progress revealed changes in age group of people. Basically, able to work people (20 to 50 years old) were ill.
In 2012, 23366 persons were sick with flu, and 3969377 persons had SARS. The peak was in March-April, 2012 the highest level of sick rate was recorded in March-15819 cases. The biggest part of the population with flu was children-144666 cases. The children were the basic part of the population with SARS-4624437 cases. In territorial terms, the population of northern and east areas of Ukraine (Chernigov and Donetsk) was affected by flu the most. The highest level of SARS sick rate was recorded in east areas (Donetsk and Dnepropetrovsk) and in Kiev.
In 2013, flu and SARS had 6134755 persons, of which 233500 persons had flu. Laboratory confirmed cases of flu were found in 2908 persons. 18 persons (of them 1 child) died. SARS was recorded in 5901275 persons, of which 3866280 children. The peak was in February, 2013 [2].
During epidemiological season 2015-2016, flu sick rate increase was observed, the flu was caused by A (H1N1) virus which turned out to be aggressive, with a considerable number of serious complications (virus pneumonia) was observed. Epidemic started from southern regions of Ukraine (Odessa region) and spread on all regions of the country. As of February 9, 2016, more than 300 persons of able to work age died of flu complications in Ukraine.
The air-drop mechanism of infection transmission, high susceptibility, presence of appreciable percent of the effaced forms of illness and minimum incubation interval cause acute “explosion-like” increase of sick rate. In such conditions, nonspecific sanitary-antiepidemic measures (use of flu masks, aeration of premises, dissociation of schoolchild etc.) cannot suspend a flu epidemic and have limited efficiency. For the prevention of the flu disease, repeated attempts to use various chemical prophylactic and immune modulating medications were done. However, the necessity of repeated daily administration of such medications, side effects and short-term preventive effect encouraged the scientists of entire world to continue a search of more efficient ways of protection against flu. Since the second half of thirties years of the last century, fundamental works on creation of efficient vaccines against flu began with the purpose of active immunization as a method of long preventive action.
Now, flu prevention by means of vaccination is conventional and is supported by the experts throughout the world. The vaccination is recommended by WHO as a unique and obligatory measure of flu prevention and its consequences for the high risk group on development of complications and death as a result of flu [3,4].
High risk groups (with small differences in different countries) include:
High risk groups usually include about 20 % of total population, and the number of vaccinated persons among them fluctuates from 80% in the Netherlands, France, Belgium, and Spain to 30% in Switzerland and Great Britain [16].
The vaccination is recommended in all high risk groups, it is possible to lower essentially the number of the complications related to flu and mortality. However, it is necessary to notice that the efficiency of vaccination is a variable, and in a group of advanced age it can be lower 50%. The scientists constantly work over new methods of vaccination, aspiring to raise its efficiency. In the previous researches, preparing flu virus to get a polyvalent flu vaccine, it was established that purifying and concentrating flu virus by high-speed centrifugation method in a gradient of density of sucrose and by electrophoresis in polyacrylamide gel, the flu virus was not released from trypsin-like proteinases [3,17].
The objective of this study is to check of presence of trypsin-like proteinase and its inhibitor in flu and other vaccines and in immunobiological blood preparations of National and foreign manufacture.
The following commercial preparations have been used in this paper: “Interferon leukocytal human”, “Immunoglobulin human placental”, donor 10% (Biofarma, Kiev), gonococci vaccine (Biolec, Kharkov) herpetic vaccine (Odessa factory of bacterial preparations, Odessa), vaccines for flu prevention, season 2002/2003 “Influvac” which consists of hemagglutinins and neuraminidase of flu virus strains: А/Moscow/10/99 H3N2), А/New Caledonia/20/99 (H/N), B/Hong Kong/330/2001 (Solvay Pharmaceuticals B.V. The Netherlands), “Fluarix” which consists of hemagglutinins of strains (H1N1) A/New Caledonia (H3N2), А/Panama and В/Shandont 17/97 (Smith Klein Beecham Biologicals, Belgium) and “Vaxigrip” who consists of three strains of flu virus (Pasteur Merier Connot, France), hepatitis vaccine A-“Avaxim” (Pasteur Merier Connot, France), blood preparation received from heparin (antifactorXa)-“Fraxiparine” (Sanofi- Сhinoin, France), calf blood preparation for hemodialysis “Solcoseryl” (Solco, Switzerland). The preparations were studied before the expiration of their shelf life. All preparations have been bought in drugstores of Odessa.
The activity of tripsyn-like proteinase and its inhibitor were determined by A. Levitsky’s method in our modification [4], total protein by O. Lowry’s method [18].
As it is evidenced by the results of the researches presented on figure 1, in all studied commercial preparations (human immunoglobulin, leukocytal interferon, herpetic vaccine, gonovaccine and tularemic vaccine manufactured by national industry), the presence of trypsin-like proteinase has been found in interferon, immunoglobulin and herpetic vaccine. In tularemic vaccine and gonovaccine, trypsin-like proteinase has not been found. Thus, presence of inhibitor and trypsin-like proteinase is not obligatory in all blood preparations, and their presence is peculiar to antiviral vaccines and preparations.

Figure 1: Activity of trypsin-like proteinase and protein extinction in the
commercial preparations received from donor blood.
Note: 1 - Immunoglobulin; 2 - Interferon; 3 – Herpetic vaccine; 4 -
Gonovaccine; 5 - Tularemic vaccine
The presence of trypsin-like proteinase and its inhibitor (Figure 2) has been found in human placental immunoglobulin, leukocytal interferon, and herpetic vaccine. The biggest activity of inhibitor of trypsin-like proteinase was established in a human placental immunoglobulin (60,185 c.u.). The inhibitor of trypsin-like proteinases also has been found out in interferon (15,79 c.u.) and in herpetic vaccine (19,750 c.u.). Tularemia vaccine contained only traces of trypsin-like proteinase inhibitor activity (0,544 c.u.) (Figure 2). In gonovaccine, nor inhibitor neither proteolitic activity have been found. Hence, immunoglobulin preparations have the highest activity of inhibitor, and the preparation of antibacterial vaccines is accompanied by their reduction.
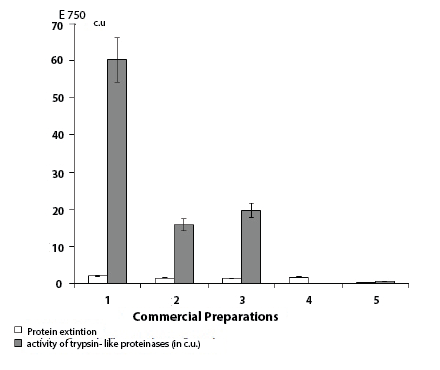
Figure 2: Activity of an inhibitor of a trypsin-like proteinase and a
proteins extinction in the commercial preparations received from a donor
blood of the human.
Note: 1 - Immunoglobulin; 2 - Interferon; 3 - Herpetic vaccine; 4 -
Gonovaccine; 5 - Tularemic vaccine
In immunoglobulin, the activity of trypsin-like proteinase inhibitor was 4,0 times more than in interferon.
On the one hand, the established fact evidences protein pollution of the preparations received from donor blood and insufficient purity of gamma-globulin produced in industrial way. On the other hand, the determination of proteolytic activity of trypsin-like proteinases and the activity of trypsin-like proteinase inhibitor can serve as a marker of preparation purity. It is possible to assume that the system proteinase/ inhibitor is somehow functionally linked to antibodies.
Thus, the commercial immunoglobulin preparation contained the highest activity of trypsin-like proteinase inhibitor in comparison with other preparations that, in our opinion, causes a part of protective effects in many serious infectious diseases. On the other hand, those blood fractions, which immunoglobulin is derived from, can be considered as a source for inhibitor.
The results of our studies allowed us to assume that, apparently, national preparations are not sufficiently purified from protein.
Preparations of foreign firms also contained an inhibitor and trypsinlike proteinase. And the quantity of an inhibitor in 250 times exceeded the content of proteinases (Table 1). The inhibitor Greatest quantity became perceptible in anti flu vaccine of “Influvac” (180,86 g/l), “Fraxiparine” (111,30 g/l) is slightly less in a preparation. The least quantity of inhibitor trypsin-like proteinases was contained by a preparation of “Avaxim” (inactivated vaccine against hepatitis).
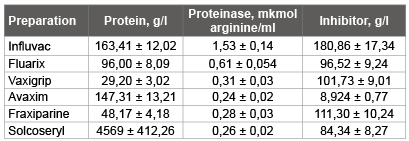
Table 1: Presence of a proteinase and its inhibitor in commercial immunobiological the preparations which are let out by firms of foreign countries (n=3)
From these three anti influenzal vaccines it is possible to consider that “Vaxigrip” has the greatest efficiency, since it contains the least quantity trypsin-like proteinase and a considerable quantity of the inhibitor influencing, in our opinion, on formation of protective forces of an organism while the proteinase can promote development of a virus infection.
Vaccines of “Influvac” follow to put on last place on these indicators, since it contains the greatest quantity of trypsin-like proteinases. However, on I. Chaloupka and co-workers [19,20]. This vaccine has the high maintenance of an ovalbumin and among often applied vaccines yields the best results [10,12].
“Avaxim” (the vaccine against a hepatitis), consists of strain GBМ of a virus of a hepatitis A (160 antigenic units) and are contains the least quantity of an inhibitor and a proteinase in comparison with other vaccines.
The blood preparation “Fraxiparine” consists of fragmented elements glycosaminoglycan a heparin (9500 МЕ). Active substance is nadroparin calcium-the low-molecular heparin received from a standard heparin by a method of a depolymerization in special conditions (the given instructions). Under our data “Fraxiparine” contains an inhibitor considerable quantity trypsin-like proteinases and the extremely insignificant quantity of proteinase. According to the instruction “Fraxiparine” it is characterised by the expressed activity concerning the factor of Ha. J. Nagai and co-workers [21] consider that the factor of Ha splits a hemagglutinin of a virus of flu on the only one segment of arginine, on two subunits - НА1 and НА2. The virus-activating proteinase and the factor of Ha not only structurally, but also are functionally similar each other [21]. On classifications Boehringer Mannheim Biochemica, the factor of Ha concerns to serine proteinases. Hence, “Fraxiparine” it is possible to consider as an inhibitor trypsin-like proteinases.
“Solkoseryl” consists of deproteinized calf blood extract and contains a wide spectrum of natural low-molecular substances. Under our data “Solkoseryl” has an inhibitor in significant amount, little proteinases and a considerable quantity of the general protein.
Proceeding from the above-stated, it is possible to conclude that foreign preparations are not cleared on 100% of impurity or it is impossible to separate virus protein from cell components. Viral proteins are strongly associated with cell components, therefore structure of a virus of a flu it is necessary to survey taking into account its interaction with cellular enzymes and their inhibitors.
Determination proteinases and inhibiting activity during manufacturing of commercial preparations, can serve as a marker of quality of cleanliness of a preparation.
Download Provisional PDF Here
Article Type: Research Article
Citation: Divocha VA (2016) Presence of the Owner’s Cellular Components in Vaccines and Immunobiological Blood Preparations. Int J Vaccine Immunizat 2(2): doi http://dx.doi.org/10.16966/2470- 9948.112
Copyright: © 2016 Divocha VA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access