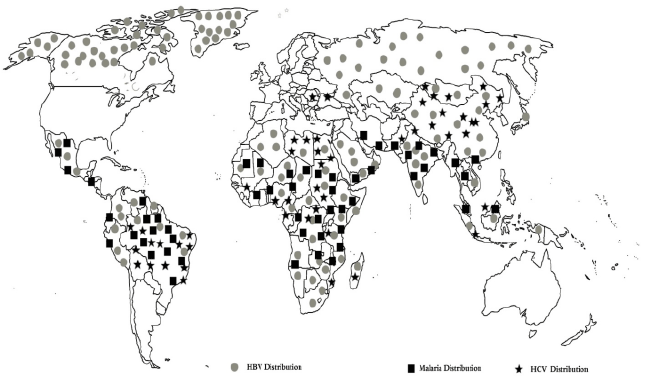
Figure 1: Showing HBV, HCV and malaria distribution
Gasim I Gasim1 Ishag Adam1,2*
1Qassim College of Medicine, Qassim University, Saudi Arabia*Corresponding author: Ishag Adam, MD, PhD, Faculty of Medicine, University of Khartoum, P.O. Box 102, Khartoum, Sudan, Tel: +249912168988, E-mail: ishagadam@hotmail.com
Background: Malaria remains a major health threat worldwide. Endemic regions for malaria are endemic for other infectious diseases that might affect the malaria infection.
Methods: A systematic search was conducted where it included published data about HBV, HCV and malaria. Published data on epidemiology, pathogenesis and consequences of HBV, HCV and malaria, were extracted from relevant studies. Epidemiology of co-infection has not been well studied, and studies in this concern will definitely draw the attention of decision makers towards such problem.
Results: Younger age and male gender were risk factors for co-infection. There were no protective effects of HBV vaccine against malaria. The interaction between malaria parasites and HCV among chronic HCV carriers might slow the emergence of the former and that could help in determining new therapeutic approaches to defeat malaria.
Conclusion: Strategies to improve currently available diagnostic techniques, researches dealing with therapeutic and prophylactic agents and protocols, vector control procedures, vaccine bringing up evolution, and other operational tools and approaches are needed.
Malaria; Endemic; Hepatotropic; Vaccine; Hepatitis B
Malaria remains a major health threat worldwide. Endemic regions for malaria are also endemic for other infectious diseases that might affect the malaria infection [1]. Examples for such a common endemic infection sharing the same territory with malaria are hepatitis B virus (HBV) and hepatitis C (HCV) [2-4]. HBV stimulates a potent proinflammatory Type 1 immune response (Th1), which is of paramount importance for Plasmodium clearance; however, it is also incriminated in disease severity [5]. Whilst challenging, data on the effects of HBV on the clinical presentation of malaria are scarce. Pasquetto et al. demonstrated in a mice model that intra hepatic HBV replication is inhibited by P. yoelii infection [6], moreover, production of interferon (IFN)-c and IFN-a/b is increased in the liver. In humans, information from a small study proposes that acute P. falciparum malaria alters HBV viremia in patients with chronic HBV infection [7]. Moreover, a study carried in Vietnam illustrated that patients with cerebral malaria had a slightly greater vulnerability to demonstrate HBV surface antigen (HBsAg) sero-positivity [8]; nevertheless, the aforementioned study failed to show any significant relation between the overall risk of death caused by severe falciparum malaria and positivity for HBs Ag [8]. However, there is lack of strong evidence supporting the suggestion that the clinical status of underlying hepatitis B-related liver disease is affected during malaria infection. On speaking about hepatitis C, Ouwe-Missi-OukemBoyer found an interaction between malaria parasites and HCV among chronic HCV carriers leading to slower emergence of the former [9]. Furthermore, having the three infections sharing an intra-hepatic stage as part of their life cycles, interactions between the three pathogens have been proposed to occur at both immunological and cellular levels, not only this but on looking at their epidemiological maps, a clear intersection is seen between the areas of endemicity of the three pathogens, please see Figure1. Such interactions between HBV and malaria have already been demonstrated in a mice model [8]. It is intriguing, that all three pathogens may also utilize common receptors amid the hepatocyte invasion [10- 12]. Furthermore, the impact of HBV and HCV infection on the clinical picture of malaria has not been adequately addressed. In this review, we aimed at putting together published data and analyzing it in order to come out with a clear picture about the pathophysiology, clinical presentation, and future prospects.
Asystematic search was conducted where it included published data about HBV, HCV and malaria. Published data about epidemiology, pathogenesis and repercussions of HBV, HCV and malaria, were extracted from relevant studies. The databases were searched using the words “Hepatitis B virus”, ‘Hepatitis C Virus” , “malaria HCV co-infection”, “epidemiology”, “Africa” , “South America”, “Asia” and occasionally, names of particular countries where entered interchangeably utilizing different search engines such as MEDLINE, Pubmed, MiPc library and Google.
Epidemiology of hepatitis B virus and malaria co-infection has not been well studied; some studies found that co-infection existed among about 41% out of 337 blood donors [13], nevertheless, the study was weak in methodology where no obvious inclusion or exclusion criteria were set, furthermore, the was not among the general population. Another study found the prevalence among Brazilian general population was as high as 1.8% [14]. Omalu et al. found a prevalence of 7.8% among a group of pregnant Nigerian women [15], this study was also defective in methodology as no clear criteria for selection or exclusion were set, and the pregnant women might be more vulnerable than the general population. On the other hand a study conducted by Pakistani investigators found no evidence of co-infection between HBV and malaria nor did they found any evidence of co-infection between the latter and hepatitis C virus [16], however their study was carried in a hypo-endemic endemic area for malaria [17]. Nevertheless, there is no studies on HCV and malaria co-infection., Younger age of patients has been mentioned by a number of researchers as risk factors especially among pregnant women [13,14].

Figure 1: Showing HBV, HCV and malaria distribution
Pasquetto et al. described the immunological features of Malaria and HBV co-infection, where they described that the hepatic stage infection seemed to trigger an early T cell–independent cytokine response along with a delayed cytokine response that was simultaneous with the infiltration of T cells. On the other hand, the T cell response appeared earlier in the blood stage than in hepatic stage infection, possibly because parasitemia was detectable earlier in those animals and a T cell–independent phase was not seen, perhaps reflecting that it was induced primarily by infected hepatocytes. In both cases, however, the generated cytokine ripostes were accompanied with a decline in HBV RNA and DNA in the liver [6]. Both HCV and malaria infections use common host factors like HSPGs, CD- 81, SR-B1, and ApoE [12].
Andrade et al found (among 636 Brazilian patients) that HBV infection was associated with a decreased intensity of malaria infection among individuals in the study [18]. They proposed that this effect is due to cytokine balance and control of inflammatory ripostes [18]. In contradistinction, another group of researchers concluded that HBV and malaria do not seem to significantly affect each other and evolve independently [19], nevertheless, the study was a hospital based study with the bulk of patient being females. A third opinion stated that the immune response against falciparum infected red blood cells might be suppressed by HBV carrier status [20], disappointingly, this study lacks a clear methodology. However, immune deficient responses to both infections might take place in some subjects leading to concomitant lower immunity against falciparum infected red blood cells along with incapacity to clear HBV [20]. The interaction between malaria parasites and HCV among chronic HCV carriers has been found to slow the emergence of the former [9].
HBsAg has been considered an integral part of Malaria vaccine (RTS, S, which is composed of a hepatitis B virus (HBV) surface antigen (HBsAg) including the repeat region and C terminus of P.falciparum CS protein (amino acids [aa] 207 to 395))a vaccine that has already gone through phase I and II trials, and showed sterile protection, i.e., total absence of detect able blood-stage of malaria infection, in about 41% of immunized volunteers [21-26]. On the other hand, studies conducted to assess the protective effect of HBV vaccine against malaria found no evidence [27,28].
As elimination of malaria is a global aim, supplementary tools are required, such as vaccination, in order to provide long-term prevention [29]. Such strategies predict improving currently available diagnostic methods, researches dealing with therapeutic and prophylactic agents and protocols, vector control procedures, vaccine bringing upevolution, and other operational tools and approaches [29]. The interaction between malaria parasites and HCV among chronic HCV carriers has been found to slow the emergence of the former a thing that could help in determining new therapeutic approaches to defeat malaria [9].
Epidemiology of co-infection has not been well studied, and studies in this concern will definitely draw the attention of decision makers towards such problem. Younger age and male gender were found to be risk factors for co-infection. No protective effects for HBV vaccine against malaria were found. The interaction between malaria parasites and HCV among chronic HCV carriers has been found to slow the emergence of the former a thing that could help in determining new therapeutic approaches to defeat malaria. Strategies to improve currently available diagnostic techniques, researches dealing with therapeutic and prophylactic agents and protocols, vector control procedures, vaccine bringing up evolution, and other operational tools and approaches are needed.
The authors declare that they have no competing issues of interests.
The authors would like to acknowledge the efforts carried by Engineer Ali Kamal Ali for his great help with the figure included
Download Provisional PDF Here
Aritcle Type: Mini Review
Citation: Gasim GI, Adam I (2015) Hepatitis B Hepatitis C virus and Malaria co-infection. Int J Vaccine Immunizat 1(1): doi: http://dx.doi. org/10.16966/2470-9948.101
Copyright: © 2015 Gasim GI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access