Introduction
After allogeneic hematopoietic stem cell transplantation (HSCT), we are often confronted with acute renal failure (ARF) and sometimes this complication is severe. The median time of onset of ARF after allogeneic HSCT was between 7 and 40 days according to several previous studies [1- 5]. Pre-existing kidney dysfunction, arterial hypertension, conditioning with total body irradiation (TBI), sepsis, acute graft-versus-host disease (aGvHD) and microangiopathic syndromes as sinusoidal occlusion syndrome may be associated with renal impairment. Acute renal failure may also occur in the absence of these conditions due to nephrotoxicity of many drugs, such as amphotericin B or cyclosporine (CsA) which are the most frequent [1,4-5].
Calcineurin inhibitors had adverse effect on the renal afferent arteriole with vasoconstriction associated with an imbalance between vasoconstrictor (endothelin and thromboxane) and vasodilator agents (prostaglandin E2, prostacyclin and nitric oxide). Concomitantly the inhibition of the calcineurin-NFAT signaling leads to COX-2 inhibition associated with renal vasoconstriction and a decrease of glomerular filtration rate (GFR). Adequate monitoring of serum creatinine and the serum residual value of CsA or tacrolimus is crucial for detecting nephrotoxicity induced by these drugs; an increase in concomitant serum creatinine and residual level of CsA or tacrolimus will allow to be stopped or adjusted the dose of CsA or tacrolimus [6-8]. The renal toxicity of calcineurin inhibitors is the most common cause of ARF after allogeneic HSCT.
Tacrolimus is now available in two oral formulations: standard-release (Prograf™, twice-daily tacrolimus) and extended-release (Advagraf™, once-daily tacrolimus). Tacrolimus extended-release (Tacrolimus ER) has been developed with the aim to obtain a stable and progressive absorption through intestinal tract over the day.
In previous studies in kidney and liver transplantation, the extendedrelease tacrolimus has been compared with the standard-release formulation to shown bioequivalent drug exposure, efficacy and safety [9-14]. Moreover, several transplantation centers showed in recent studies advantageous effects of tacrolimus in comparison with CsA regarding renal function in patients after renal and heart transplantation. [15-16].
Based on these observations, we realised a prospective study in which CsA (Neoral Sandimmun, Novartis Pharma) is replaced by tacrolimus ER (Advagraf, Astellas Pharma) in case of renal impairment secondary to CsA nephrotoxicity. Discontinuation of nephrotoxic drugs and correction of dehydration were realised before the switch of molecule. Renal relapse of malignant disease was excluded and other cause of renal failure was excluded to.
Patients, Method
We enrolled 30 consecutive patients in a pilot study between March 2012 and March 2016 from two centers in France (Lyon and Nancy) with renal impairment due to CsA. Renal impairment was defined by a serum creatinine>90 µmol/L. All patients received an allogeneic HSCT for hematologic malignant disease after myeloablative or reduced intensity conditioning (RIC). The prophylaxis of acute GvHD was provided by a combination of CsA and methotrexate or mycophenolate mofetil. The minimum age for inclusion was 17 years. To be included, patients should have normal renal function defined by serum creatinine<90 µmol/L and estimated GFR (eGFR) with CKD-EPI equation>60 ml/min prior to transplantation. The demographic and characteristics of patients are presented in table 1. After exclusion of other cause of renal failure, correction of dehydration and discontinuation of CsA, the conversion was carried out and the dose was established on an mg:mg basis 1:100 from CsA total daily dose to a total daily dose of tacrolimus. The discontinuation of CsA is followed by taking tacrolimus ER the next day at noon. In this cohort, 27 patients received oral formulation of tacrolimus ER (Advagraf) and 3 received Prograf initially iv. and then converted to tacrolimus ER oral form. The dose was readjusted to obtain a tacrolimus blood trough level between 5 and 15 µg/L. We evaluated the tacrolimus blood trough level changes after conversion, serum creatinine, potassium, one time a week from one week after switching to discontinuation. Renal function was analysed by serum creatinine levels and estimated glomerular filtration rate (eGFR) assessed by CKD-EPI equation. The stage of acute kidney injury (AKI) has been established according to definition by Acute Kidney Injury Network [17]. This study was conducted according to the 2008 ethical Declaration of Helsinki and the ethics committees of each center approved this study.
| Gender |
N=30 |
Male
Female |
21 (70%)
9 (30%) |
| Median age (range) |
54 (17-67) |
Diagnosis
AML
ALL
MM
MDS
MF
NHL
HL |
11 (37%)
5 (17%)
5 (17%)
4 (13%)
2 (6.5%)
2 (6.5%)
1 (3%) |
Status at transplantation
CR 1
CR 2 and more
PR
Refractory |
12 (40%)
10 (33%)
5 (17%)
3 (10%) |
Conditioning Regimen
MAC
TBI based
Bu Based
RIC
Flu Bu
Flu Mel
FLAMSA-RIC |
13 (43%)
9
4
17 (57%)
10
2
5 |
Source of stem cells
BM
PBSC CB |
10 (34%)
19 (63%)
1 (3%) |
Donor
HLA 10/10 matched related HLA 10/10 matched unrelated HLA 5/10 haplo-identical
HLA 5/6 Cord Blood |
11 (37%)
17 (57%)
1 (3%)
1 (3%) |
GVHD prophylaxis
CsA + MTX
CsA + MMF |
13 (43%)
17 (57%) |
Table 1: Characteristics of patients and transplantation
Abbreviations: AML=acute myeloblastic leukemia; ALL= acute lymphoblastic leukemia; MM= multiple myeloma; MDS= myelodysplastic syndrome; MF=myelofibrosis; NHL=non Hodgkin’s lymphoma; HL=Hodgkin’s lympoma; CR= complete response; PR=partial response; MAC=myeloablative regimen; TBI=total body irradiation; Bu=busulfan; RIC=reduced intensity conditioning; Flu=fludarabine; Mel=melphalan; BM=bone marrow; PBSC=peripheral blood stem cells; CB=cord blood; GvHD=graft versus host disease; CsA=ciclosporine; MTX=methotrexate; MMF=mycophenolate mofetyl.
Statistics
The exact tests of Wilcoxon Mann-Whitney and Kruskal Wallis (nonparametric tests) were performed for the physiological parameters analysis. The p values are calculated with the Mann-Whitney tests.
Results
The characteristics of patients and transplantation are summarized in table 1.
All patients received allogeneic HSCT for malignant blood disease; the median age in this cohort was 54 years (range, 17-67). Before the switch, 22 patients (73%) had CsA for GvHD prophylaxis and 8 patients (27%) for acute GvHD treatment in association with prednisone.
After transplantation, the median follow-up was 35.6 months (range, 1-51,7).The median time to which tacrolimus replaced cyclosporine was 40 days (range, 3-1286) and the median of serum creatinine for switching was 110 mol/L (range, 94-262). Concerning creatinine and potassium levels, the median level was 105 µmol/L (range, 51-262) with cyclosporine and 84 µmol/L (range, 50-129) with tacrolimus for creatinine (p<0.01) (Figure 1) and 4.2 mmol/L (range, 3.2-5.2 ) with cyclosporine and 4.0 (range, 3-5.1) with tacrolimus for potassium (p<0.001) (Figure 2).
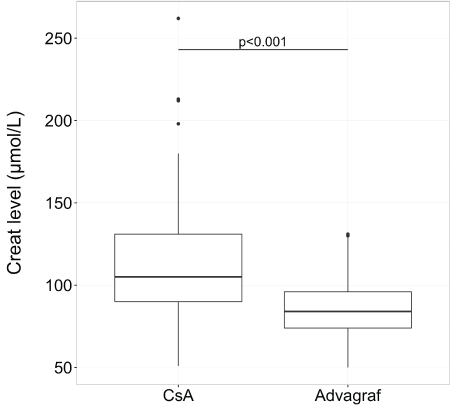
Figure 1: Creatinine level in box plot according to CsA or tacrolimus extended-release (Advagraf): creatinine level was significantly lower with tacrolimus ER (the median level was 105 µmol/L (range, 51-262) with cyclosporine and 84 µmol/L (range, 50-129) with tacrolimus ER)
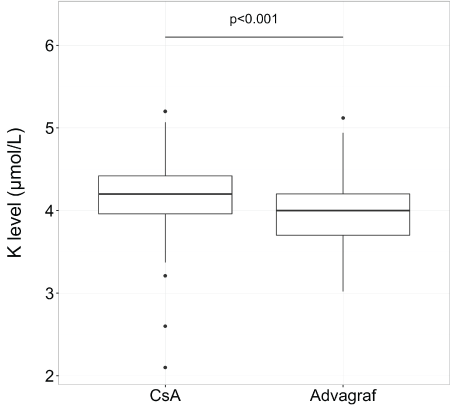
Figure 2: Potassium level in box plot according to CsA or tacrolimus extended-release (Advagraf): potassium level was significantly lower with tacrolimus ER (the median level was 4.2 mmol/L (range, 3.2-5.2) with cyclosporine and 4.0 (range, 3-5.1) with tacrolimus ER)
Before the switch, the median of serum creatinine level was 94 µmol/L (range, 51-213), 106 µmol/L (range, 57-262) and 110 µmol/L (range, 75-180) at D+30, D+45 and D+60 respectively after transplantation for patients with CsA and the median residual value of CsA was 250 µg/l (range, 162-629) at D+30 . The median dose of CsA was 200 mg (range, 25-600) and the median dose of tacrolimus ER was 2.4 mg (range, 0.5-5). The median of serum creatinine level was 88 µmol/L (range, 59-130), 84 µmol/L (range, 54-129) and 84 µmol/L (range, 54-129) at D+5, D+15 and D+30 after the switch for tacrolimus ER and the median residual value of tacrolimus ER was 8.7 µg/l (range, 2.7-15) at D+20 after the switch (Figure 3). The results with creatinine and eGFR data are summarized for each patient in table 2. Prior to transplantation, all patients had creatinine clearance>60 ml/min. The stage of renal failure prior the conversion was 1 for 16 (53%) patients, 2 for 6 (20%) patients and 3 for 2 (7%) patients. Six (20%) patients did not have the definition of acute renal failure according to acute kidney injury network (17). After 30 days of switching, all patients had creatinine clearance > 50 ml/min.
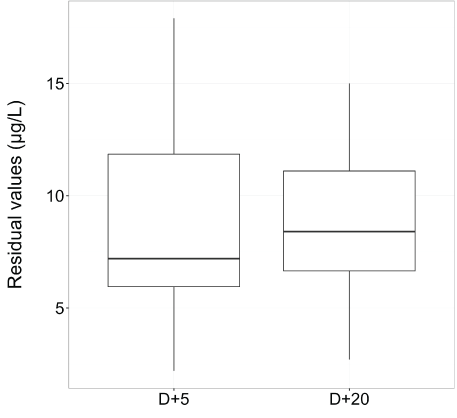
Figure 3: The residual value of tacrolimus ER was in therapeutic range (5-15 µg/l) after five and twenty days of introduction
| Patient |
Age and gender |
Creatinine and eGFR at D-30 |
Creatinine and eGFR at D+30 |
Creatinine and eGFR at D+45 |
Day of switch |
Creatinine and eGFR at day of switching |
Stage of AKI |
Creatinine and eGFR after 30 days of switching |
| 1 |
46 M |
60 and 115 |
90 and 88 |
95 and 82 |
+45 |
|
1 |
63 and 112 |
| 2 |
66 M |
68 and 95 |
85 and 82 |
262 and 21 |
+45 |
|
3 |
50 and 107 |
| 3 |
60 M |
71 and 97 |
70 and 98 |
80 and 92 |
+139 |
100 and 70 |
1 |
74 and 95 |
| 4 |
59 M |
85 and 86 |
|
|
+10 |
125 and 54 |
1 |
88 and 82 |
| 5 |
52 M |
80 and 98 |
80 and 98 |
82 and 94 |
+60 |
143 and 48 |
1 |
88 and 87 |
| 6 |
52 M |
90 and 84 |
|
|
+19 |
120 and 60 |
0 |
129 and 51 |
| 7 |
51 F |
50 and 108 |
121 and 45 |
|
+30 |
|
2 |
74 and 81 |
| 8 |
67 M |
80 and 88 |
85 and 81 |
110 and 60 |
+45 |
|
1 |
87 and 79 |
| 9 |
54 F |
65 and 93 |
156 and 32 |
|
+30 |
|
2 |
66 and 92 |
| 10 |
42 F |
50 and 115 |
84 and 74 |
88 and 70 |
+60 |
96 and 63 |
1 |
54 and 112 |
| 11 |
54 M |
50 and 117 |
51 and 118 |
57 and 111 |
+1286 |
134 and 51 |
2 |
77 and 98 |
| 12 |
30 M |
70 and 120 |
80 and 114 |
82 and 110 |
+60 |
113 and 75 |
1 |
75 and 117 |
| 13 |
55 F |
70 and 84 |
213 and 22 |
|
+30 |
|
3 |
77 and 75 |
| 14 |
29 M |
85 and 106 |
|
|
+3 |
134 and 61 |
1 |
110 and 78 |
| 15 |
29 M |
92 and 96 |
|
|
+13 |
102 and 85 |
0 |
94 and 94 |
| 16 |
57 M |
70 and 100 |
170 and 38 |
|
+30 |
|
1 |
88 and 84 |
| 17 |
57 F |
65 and 91 |
65 and 91 |
|
+40 |
112 and 47 |
1 |
90 and 61 |
| 18 |
31 M |
75 and 116 |
116 and 72 |
|
+30 |
|
1 |
87 and 102 |
| 19 |
55 M |
70 and 101 |
|
|
+15 |
129 and 53 |
1 |
67 and 103 |
| 20 |
58 F |
65 and 90 |
|
|
+14 |
94 and 58 |
1 |
76 and 75 |
| 21 |
49 M |
60 and 112 |
126 and 57 |
|
+30 |
|
2 |
61 and 111 |
| 22 |
66 M |
90 and 76 |
122 and 53 |
|
+30 |
|
1 |
96 and 71 |
| 23 |
23 F |
47 and 113 |
90 and 62 |
|
+40 |
95 and 61 |
1 |
57 and 106 |
| 24 |
53 M |
80 and 97 |
85 and 90 |
112 and 64 |
+45 |
|
0 |
80 and 97 |
| 25 |
37 M |
62 and 120 |
87 and 98 |
131 and 59 |
+45 |
|
2 |
66 and 117 |
| 26 |
55 M |
85 and 88 |
89 and 84 |
|
+41 |
105 and 65 |
0 |
89 and 84 |
| 27 |
57 F |
55 and 100 |
119 and 44 |
|
+30 |
|
2 |
70 and 83 |
| 28 |
53 M |
60 and 109 |
88 and 86 |
118 and 60 |
+45 |
|
1 |
65 and 106 |
| 29 |
17 F |
80 and 94 |
|
|
+9 |
105 and 67 |
0 |
82 and 91 |
| 30 |
52 M |
80 and 98 |
108 and 68 |
|
+30 |
|
0 |
80 and 98 |
Table 2: Details for each patient in the study: age, gender (M/F), stage of acute kidney injury (AKI), creatinine (µmol/L) level and eGFR (CKD-EPI) ml/min/1, 73 m2 at Day -30, at Day +30, at Day +45, at Day of switching if different than Day+30 or Day+45 and at thirty days after the conversion for tacrolimus ER
Abbreviations: eGFR=estimated glomerular filtration rate; AKI=acute kidney injury
Sixteen patients (53.3%) developed aGvHD grade ≥ II; for 14 patients, aGvHD was resolutive with an association of tacrolimus and prednisone or tacrolimus and extracorporeal photopheresis (ECP). Two patients receive also now treatment with tacrolimus ER and ECP for one patient and tacrolimus ER associated with prednisone for the second. The cumulative incidence of grade II-IV aGvHD was 53% (95%CI, 25-64) at+90 days and the cumulative incidence of cGvHD was 26.5% (95%CI, 7.75-45) at 1 year, 32.14% (95%CI, 11.6-52) after 2 years and 32.14% (95%CI, 11.6–52) after 3 years. Seven patients (23.3%) developed chronic GvHD with NIH score ≥ 2 after discontinuation of GvHD prophylaxis. The cGvHD was resolutive for 4 patients and 3 patients currently perform ECP or take ruxolitinib and everolimus respectively. In this cohort, 26 patients (86.6%) are alive and 25 patients (83.3%) are in complete response at the last follow-up. Four patients (13.3%) died two from relapse, one from cerebral bleeding and one from severe pneumonia (fungal and Pneumocystis jiroveci infection).
Discussion
There is currently no recommendation in allogeneic HSCT if a patient has acute renal failure secondary to CsA. Each center has its own strategy after discontinuation of CsA, most of the time, CsA is replaced by corticosteroids and CsA is resumed after normalization of renal function if possible otherwise the corticosteroids are being prosecuted. To our knowledge, this study with tacrolimus ER is the first in allogeneic HSCT. Our principal objective was to measure the serum creatinine level and the eGFR after the change for tacrolimus ER in case of decreased renal function due to CsA. Only patients with acute renal failure secondary to CsA toxicity were considered in this pilot study. For the majority of patients (53%), the stage of renal insufficiency was 1 but 2 patients had a severe renal failure with a score of 3 according to acute kidney injury network [17]. We found for creatinine a median level of 105 µmol/L (range, 51-262) with cyclosporine and 84 µmol/L (range, 50-129) with tacrolimus (p<0.01). The median of serum creatinine level was 88 µmol/L (range, 59-130), 84 µmol/L (range, 54-129) and 84 µmol/L (range, 54- 129) at D+5, D+15 and D+30 after the change for tacrolimus ER. After 30 days of switching, all patients had creatinine clearance >50 ml/min. The serum creatinine level and the eGFR are significantly improved after the change for tacrolimus ER. These results confirm previous studies in organ transplantation concerning renal protection with tacrolimus ER especially in heart transplantation. Two studies reported that CsA was associated with decreased renal function mostly in the first 6 months after heart transplantation when high CsA drug levels are imperative to prevent acute rejection of the graft; at this time a superior rejection profile of the tacrolimus based immunosuppressive regimen had been demonstrated with respect to renal function [15,18].
Concerning, the potassium level, we found an improvement in the potassium level which was often high with CsA. This observation has already been made in the past and several studies are showed that decreased numbers of mineral corticoid receptors lead to hyperkaliemia with aldosterone resistance in patients treated with CsA [6].
In healthy volunteers, acute reduction in GFR is attenuated with tacrolimus and tacrolimus ER compared with CsA and moreover tacrolimus has a lower nephrotoxicity than CsA in studies and animals and humans [6,19].
In organ transplantation, the choice between Tacrolimus (Prograf or Advagraf) and CsA is based on the experience and preference of the physician as well as the toxicity profile of the molecule according to the patient. There are several multi-centers studies and retrospective analysis in favour of tacrolimus over CsA concerning renal function in liver, heart and renal transplantation. Tacrolimus was associated with significantly lower serum creatinine levels compared to Csa and better graft survival in long term after renal transplantation. In case of renal graft dysfunction, a switch from CsA to tacrolimus was associated with a significant improvement in renal function [16,20-23]. In liver transplantation, several centers reported improved kidney function, improved lipid profile and better blood pressure [24].
In allogeneic HSCT, calcineurin inhibitors (CNIs) are habitually combined with methotrexate in myeloablative allogeneic HSCT and are usually used with mycophenolate mofetil (MMF) after reduced intensity conditioning (RIC) for GvHD prophylaxis. Cyclosporine has been used for 1983 in the prevention and treatment of GvHD after allogeneic HSCT. Concerning CsA, this molecule can cause ARF, but it is known that levels of residual values are not always correlated with the occurrence of ARF [25]. Tacrolimus has been used since the early 1990s in organ transplantation and has an advantage particularly in the prophylaxis of GvHD in unrelated allogeneic HSCT. It can also be used in case of development of GvHD with CsA prophylaxis. [26-27].
The immunosuppression secondary to CsA or tacrolimus results from inhibition of calcineurin, a calcium- and calmodulin-dependent phosphatase (protein phosphatase 3). Two different molecules are binded intracellularly by CsA and tacrolimus: cyclophylin and FKBP12 respectively. The phosphatase activity of calcineurin is inhibited by competitive binding of cyclosporine-cyclophylin and tacrolimus-FKBP12 complexes. By this mechanism, the transcription of IL-2 is inhibited and the nuclear factor of activated T cells (NFAT) which regulates IL-2 transcription is impaired. The T cell activation is therefore no effective [28-30]. In this way, these molecules allow the prevention of acute GvHD immediately after allogeneic HSCT. In our study the cumulative incidence of grade II-IV aGvHD was 53% (95%CI, 25-64) at +90 days and the cumulative incidence of cGvHD was 26.5% (95%CI, 7.75-45) at 1 year, 32.14%(95% CI, 11.6-52) after 2 years and 32.14% (95% CI, 11.6–52) after 3 years. These results are expected but the small number of patients limits the conclusions.
The main interest of this study is to demonstrate the feasibility of this molecule change in case of renal failure due to CsA without excessive incidence of GvHD or side effects. In this way, the pursuit of a calcineurin inhibitor is possible and corticosteroids should not be used, which is preferable for the prevention or treatment of GvHD and for limiting side effects. The principal limitation is the low number of patients and a prospective randomized study is required to answer the question definitely.
In conclusion, the change for tacrolimus ER was associated with significant improvement in renal function with stable tacrolimus blood trough levels. Based on these observations, we suggested that the use of tacrolimus ER in case of renal impairment secondary to CsA is safe in allogeneic HSCT patients. We hope now to conduct a large randomised study comparing CsA and tacrolimus ER through the centers from the SFGM TC (Société francophone de greffe de moelle et de thérapie cellulaire) in the aim to confirm these observations.
Authorship
MD, CP and FB participated in research design.
MD wrote the manuscript.
SM carried out the statistics.
MD, MM, FB and SM analyzed the data.
FB and MM reviewed the manuscript.
MD, CP, FB, AP, GRG, MD’AP, AN, ST, MTR and VC participated in the performance of the research.
VS, NV, CR, MM and SC contributed new reagents or analytic tools.
Disclosure
The authors declare no conflicts of interest
Funding
No funding was attributed to this study.
Abbreviations
Acute graft-versus-host disease: aGvHD
Acute renal failure: ARF
Calcineurin inhibitors: CNIs
Cyclosporine: CsA
Estimated glomerular filtration rate: eGFR
Extracorporeal photopheresis: ECP
Glomerular filtration rate: GFR
Graft-versus-host disease: GvHD
Hematopoietic stem cell transplantation: HSCT
Interleukin 2: IL-2
Mycophenolate mofetil: MMF
Reduced intensity conditioning: RIC
Tacrolimus extended release: Tacrolimus ER
Total body irradiation: TBI




