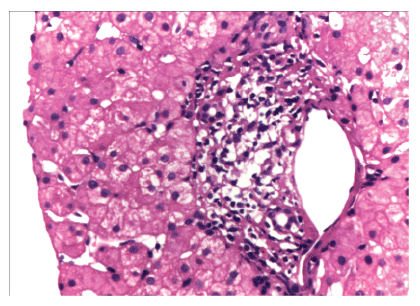
Figure 1: Liver biopsy on high power view showing mild chronic infalmmation

Naganathan Selvakumar1* Subash Gupta2 Neerav Goyal3 Sudeep Khanna4 Deep Shika Arora5
Junior consultant, Liver transplantation, Indraprastha apollo hospital, New Delhi, India*Corresponding author: Naganathan Selvakumar, Junior consultant, Liver transplantation, Indraprasta Apollo Hospital, 101, Sangamappartments, West enclave, Pitampura, Delhi-110034, India, Tel: +919871756756; E-mail: enselva1@gmail.com
Introduction: In the domain of living donor liver transplantation (LDLT)until now there is only one case report of Hepatitis C virus (HCV) infected individual with high RNA quantity undergoing antiviral therapy, achieving SVR and successfully donating the liver and completing a successful liver transplantation. On the other hand, in deceased donor liver transplantation (DDLT) HCV seropositive livers had been accepted as marginal donors for HCV positive recipients for a long time. This contrary was mainly because of unacceptably high recurrence rates combined with poor treatment tolerance in the interferon era. However with the recent introduction of effective oral direct acting anti-virals (DAA) against HCV this situation has changed for good. We describe the first ever case of LDLT using liver graft from a treated (SVR 12 weeks) HCV seropositive donor.
Case presentation: Our patient was a 57 years old lady from Turkmenistan who had decompensated CLD (Chronic liver disease) secondary to HCV infection. Her MELD score was 22 and CTP was 12.Since she was an overseas patient her chances of getting a cadaver organ were remote. Hence she was offered the option of LDLT while awaiting a cadaver organ. Her daughter volunteered to be her donor. However on donor workup she also had HCV seropositivity. Hence further donor workup was abandoned and was further evaluated for her HCV infection. She underwent 3 months of antiviral therapy with Sofosbuvir+Pegasys+Ribavirin. SVR (sustained viral response) was obtained after 12 weeks of DAA based treatment. Since the family could not find a suitable donor she continued her donor workup which included a liver biopsy. The Liver biopsy revealed preserved parenchymal architecture with mild chronic inflammatory cells. After discussion in the multidisciplinary meeting LDLT was done with modified right lobe from this donor. Post operative course in donor and recipient were uneventful. At 75 days follow up both patient and donor are doing well.
Discussion: Till now there are no proven reports to suggest that HCV seropositive donor livers have poor outcomes compared to the normal livers. Hence they are accepted in HCV positive recipients and in severely ill in DDLT. There is only one case report of LDLT with HCV seropositive donor in the interferon era [1]. With the introduction of more potent DAAs the treatment outcomes are much better [2]. We believe that this could change the thought process in accepting HCV seropositive donors there by increasing the donor pool.
Conclusion: The recent introduction of high potency anti-virals has changed the scenario of HCV seropositive persons as donors in LDLT. Donor hepatectomy is safe. These liver grafts have comparable outcome as compared to seronegative grafts.
Liver transplantation (LT) is presently the gold standard procedure for decompensated CLD [3]. Bottle neck in LT is shortage of donor organs all over the world [4]. Hence Transplant surgeons across the world are constantly exploring the possibilities of increasing the donor organ pool. Different modalities practiced by them include marginal donors, DCD donors, Living donors, swaps, Dominos, ABOi, HBs Ag and HCV seropositive donors [5]. These modalities have helped improve the donor pool by 10-40%. In the eastern world where DDLT rates are dismal LDLT forms the main bulk of organ transplantation [6] Increase in the volume of cases have made various anatomical and physiological conditions which were considered as contraindications in yesteryears as operable now [7]. We are presenting one such new clinical scenario. HCV seropositive donors are accepted worldwide in DDLT for HCV seropositive recipients but not so in LDLT. The reasons are high complication rates and fewer efficacies. However with recent introduction of DAAs where compliance rates and SVR rates are very high with reduced complication rates the scenario has changed. We publish the first case report of successful LDLT involving HCV seropositive donor to a HCV seropositive recipient. We believe this will be a breakthrough in the sense that HCV infection is often familial and many patients could not get appropriate donors because of cross infection of HCV within family members. In the near future there may be acceptance of HCV seropositive donors in seronegative recipients too.
Our patient was a 57 years old Turkmenistan national who was referred to us for liver transplantation for decompensated HCV related liver disease. She was diagnosed with HCV infection in 2007 on routine examination. She underwent no treatment citing social reasons. She was physically well after that. However, in July 2015 she decompensated in the form of ascites. She was managed medically with diuretics and hepatoprotectors. Since then she had multiple episodes of ascites responding to diuretics. She came to Indraprasta Apollo hospital for further evaluation. Her evaluation revealed decompensated CLD with MELD of 22 and CTP 10/C. She had no other contraindications for LT. Since she was an overseas patient she had very less chances of getting a cadaveric graft as per human organ transplantation act of India. Hence she was offered living donor liver transplantation. Her daughter came forward to donate the liver. She was a healthy 36 years old lady with no co-morbidities. However, she used to smoke 3 cigarettes per day. She on initial screening was found to have HCV infection with GT 1a with HCV RNA of2.9 X 10 power 5. Fibroscan score was 5. Liver functions were WNL. Hence she was rejected as donor and started on antivirals (Sofosbuvir+Peg Interferon+Ribavirin) with effect from 18 July 2015. Meanwhile the family was asked to look for alternative donor. Since there were no donors the patient was placed in the waiting list for DDLT. Since then the patient was on regular follow up as outpatient with monthly labs and 3 monthly USG and serum AFP for HCC screening. She also had multiple admissions for ascitic fluid tapping and recurrent episodes of hepatic encephalopathy. Meanwhile her daughter had completed anti-virals therapy and had achieved SVR too. She was again reevaluated for liver transplantation. Her HCV RNA was negative, LFT was WNL and Fibroscan was WNL. In view of HCV infection liver biopsy was done which revealed normal liver parenchyma with preserved architecture, no steatosis or fibrosis and few insignificant chronic inflammatory cells [Figure1,2]. Other than HCV antibody positivity she had no contraindication for liver donation. CT volumetry revealed adequate remnant and good GRWR. After extensive discussion patient and the donor were taken up for LDLT.

Figure 1: Liver biopsy on high power view showing mild chronic infalmmation
Figure 2: Liver biopsy on low power view showing mild chronic infalmmation
Modified right lobe graft of 852 gms was harvested from the donor. Remnant was 33% of total liver volume. No blood or blood products were transfused. Operation time was 6 hrs. Donor was extubated on table. Donor had uneventful postoperative recovery. Peak serum bilirubin was 2.1 on POD 2 and Peak INR was 1.8 on POD 2. The abdominal drain was removed on POD 8 after a negative HIDA scan report. She was discharged home on 10th POD. At 3 months follow up she is doing well. LFT is WNL. USG has shown sufficiently regrown liver. HCV RNA is undetectable at 6 months post completion of therapy and 3 months post donor hepatectomy.
Explantation was done by Mercedes Benz incision. High hilar Portal dissection was done to preserve extra lengths of vasculobiliary structures. Anterior sector drainage was reconstructed in the back table with the help of native portal vein graft harvested from the explanted liver.
Implantation was done with Side clamping of inferior vena cava. Cold ischemia time was was 92 mins and warm ischemia time was 38 mins. Blood loss was 1000 ml. 3 units of packed red blood cells were transfused during surgery. Operation time was 8 hrs 30 mins. Patient tolerated the procedure well. Postoperatively recipient was electively ventilated overnight and was extubated next day morning as per protocol. She was started on immunosuppression with Tacrolimus, mycophenolate and steroids on first post operative day. Peak bilirubin was 5.6 on first post operative day and peak INR was 2.3 also on first postoperative day. Serial Dopplers revealed good graft inflow and outflow. Patient had smooth post operative recovery. The drains were removed on 10th day of operation. She had intensive care unit stay of 7 days and hospital stay of 18 days after the procedure. At three month follow up patient is doing well and on stable immunosuppression. As per protocol she has been started on DAAs months with Sofosbuvir and ledipasvir.
In DDLTs Hepatitis C antibody-positive donors have traditionally been considered a dilemma, because of the high risk of transmission of HCV through transplantation of any organ [8]. A positive donor HCVRNA, indicative of active viral replication, has been associated with a higher risk of transmission, but often this information is not available in the time frame required to utilize a deceased donor [8]. The risks of transmission from HCV-RNA negative, HCV antibody positive donors have not yet been fully defined. However potential risk of recurrence of virus is present. Hence HCV positive donor organs are offered to the HCV positive recipients only. On the contrary in HBV infected persons who have cleared the virus and have formed sufficient antibodies are accepted as potential donors irrespective of the viral status of the recipient [9]. This is because once the antibodies are formed the person is immune to HBV. With this background let us approach this case.
In LDLT till now there is no publication in the literature with HCV seropositive donors in the post DAA era. There is one case report from Japan in the interferon era where the individual was 7 years post treatment with interferon and had persistent SVR [1].
Traditionally the recurrence of HCV on treatment with interferon based regimens was high [10]. Side effects of treatment were also high. These may be the reasons why there are very less incidence of liver donations in the interferon era.
The introduction of newer anti-virals has revolutionized the treatment of hepatitis C for all genotypes. AASLD guidelines for HCV treatment for different genotypes with newer DAAs have been published. The response rates are very high. Recurrence rates are low. Recent introduction of interferon free regimens is still more potent [11]. Thanks to the WHO for making these medicines available in the developing countries at the manufacturing cost in many developing countries.
There is no case report in the literature involving an individual who has been diagnosed with HCV infection on donor evaluation, completing treatment attaining SVR and then completing liver donation. Though 3 months post completion of treatment looked too early and the liver biopsy one week prior to liver donation suggested chronic inflammatory cells in the porta we believe that with the viral clearance the inflammation also is bound to subside in the future. Though there is 100 % risk of the graft reinfection with HCV from the recipient we could not wait further in view of worsening general condition of the recipient. At the time of writing this article the recipient has already completed 90 days post transplantation with stable liver functions and stable immunosuppression and also has started her anti-vital treatment with Sofosbuvir+ledipasvir treatment regimen.
The successful outcome of this case has given us hope that more and more such LDLTs can be performed in the future with virus cleared HCV seropositive donors with equivalent donor safety and recipient outcomes. This case has given us hope of new source of organ donor.
The newer anti-virals have opened a fresh ray of hope for millions of families affected with HCV infection. Early outcomes of LDLT with HCV positive donor are satisfactory. Donor hepatectomy is safe in HCV seropositive and RNA negative individuals. Graft and recipient outcomes did not show any major deviation from clinical course in the first 90 days. Though this case report is about HCV to HCV living donation we hope this can extend to HCV to non HCV living donations too. The final verdict will be out only with proven long term graft and patient survival with more number of cases.
Download Provisional PDF Here
Article Type: Case Report
Citation: Selvakumar N, Gupta S, Goyal N, Khanna S, Arora DS (2016) Is it Time to Accept HCV Seropositive RNA Negative Donors in Living Donor Liver Transplantation in the Era of Oral Anti-virals Against Hepatitis C? A Case Report. Transplant Res J 1(1): doi http://dx.doi.org/10.16966/trj.105
Copyright: © 2016 Selvakumar N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access