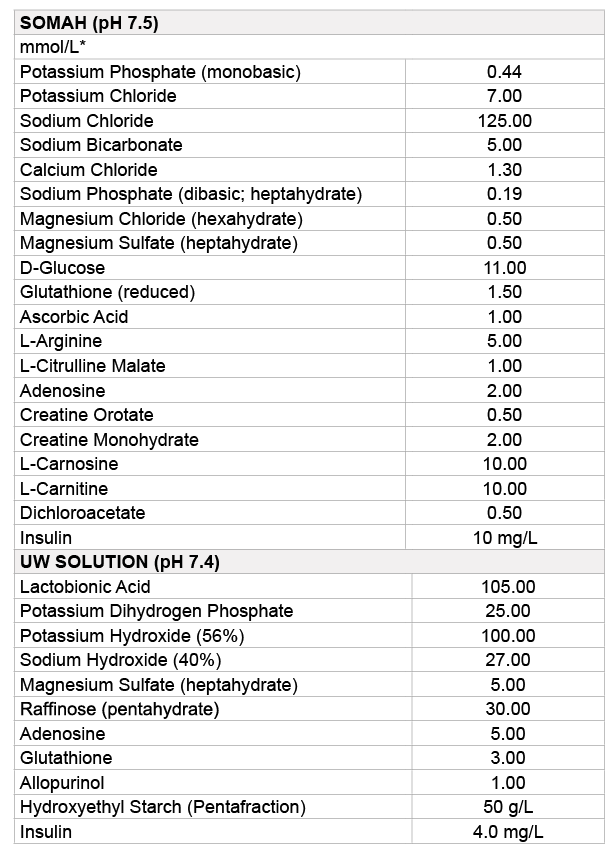
Table 1: Composition of Somah and UW solutions

Haiyan Cao1,2,3,# Samar K Lowalekar1,2,3,# Arun Chaudhury1,2,3 Xiu-Gui Lu1,2,3 Patrick R Treanor3 Hemant S Thatte1,2,3*
1Cardiothoracic Surgery Division, Department of Surgery, Harvard Medical School, Boston, Massachusetts, USA*Corresponding author: Hemant S Thatte, Department of Surgery, VA Boston Healthcare System 1400 V. F. W. Parkway, West Roxbury, MA 02132,USA, Tel: 857-203-5919; Fax: 857-203-5592; E-mail: Hemant_Thatte@hms.harvard.edu
Background: Despite increased utilization of kidneys from deceased donors (DCD; Donation after Cardiocirculatory Death) for transplantation, delayed graft function (DGF) and primary non-function (PNF) incidence remain high for DCD kidneys while post-transplant graft survival is lower. An enhanced preservation technology will facilitate qualitative improvements in DCD kidneys and outcomes. Present study evaluated the ability of novel organ preservation solution Somah compared to University of Wisconsin (UW) solution for extended storage of DCD kidneys.
Methods: Porcine DCD kidneys procured 60 ± 10 minutes after cardiac death were flushed and preserved in storage solutions at 4°C for 72 hours. Gross morphology was evaluated and biopsies taken at 1, 6, 24 and 72 hours for histopathology (HP), tissue high energy phosphates (HEP; ATP+Creatine Phosphate) and western blotting for caveolin, endothelial-nitric oxide synthase (eNOS) von-Willebrands factor (vWF) and erythropoietin (EPO). Metabolic parameters of pH, pO2, pCO2, glucose and lactate levels in solutions were determined at similar time points.
Results: Somah-stored kidneys demonstrated normal gross morphology, greater metabolic activity and preservation of energy (HEP). In contrast, UW-kidneys showed mottled appearance, a greater degree of time-dependent increase in nuclear hyperchromacity of tubular epithelial cells, attenuation of metabolism, progressive decrease in HEP, and in the expression of eNOS, vWF and EPO, indicating tissue damage during storage.
Conclusion: Better preservation of renal tissue and higher energy status of Somah-preserved kidneys suggests that DCD kidneys stored in Somah are likely to perform better upon transplantation. Future studies including transplantation of DCD kidneys stored in Somah are required for further evaluation.
Extracorporeal storage; Kidney transplantation; Preservation solution; Somah; Donation after cardiocirculatory death
CP: Creatine Phosphate; eNOS: endothelial Nitric Oxide Synthase; EPO: Erythropoietin; HEP: High Energy Phosphates; vWF: von Willebrands Factor
Eighty-six of every hundred patients requiring renal transplant last year, did not get one, adding to the ever mounting waitlist for transplantrequiring individuals [National Kidney Foundation; http://www.kidney. org]. This is despite tremendous progress in solid organ transplantations in last several decades and availability of renal replacement therapy (Hemodialysis and Peritoneal dialysis) which are not only associated with severely debilitating and/or life-threatening complications but hinder routine lifestyle of patients due to frequent requirements for hospital visits, imparting a huge financial burden on society.
While last year 14000 patients in US received renal transplant, 2500 patients are added to renal transplant waitlist each month, with around 100,000 patients awaiting kidney transplantation at the time of writing this paper; the average waiting time being three to five years [1]. Although, 65% of renal grafts are procured from deceased (DCD; Donation after Cardiac Death) donors, these grafts are twice as likely to develop delayed graft function (DGF) compared to standard- criteria donors [2], an increased incidence of primary non-function (PNF) [3], and halved overall graft survival. Whereas grafts exposure to warm and/ or cold ischemia is an obligate inevitability of solid organ transplant, the resulting cellular/tissue damage during this period is consequential of the less than desired post-transplant operative renal function. The above data indicate an enormous potential to improve quality of DCD kidneys for transplantation by making advances in preservation technology, and thus more desirable long-term patient outcomes.
To this end, we formulated a novel organ storage solution, Somah, demonstrably superior in ex-vivo static preservation of porcine cardiac grafts, compared to current clinical norms such as Celsior and UW [4-7]. The rationally incorporated components of Somah, not only help attenuate reperfusion injury (by reactive oxygen species), the synthesis of high energy phosphates (HEP; ATP+Creatine Phosphate) is also enhanced significantly, thus appropriating metabolic requirements during storage and immediately upon reperfusion. The superiority of Somah has also been demonstrable in extracorporeal static storage of DCD Livers [8] and perfused storage of DCD Lungs [9].
Our studies on porcine hearts and livers demonstrate that preservation of energy metabolism during extracorporeal storage directly correlates to superior post-storage biochemical and functional outcomes. Present study was designed to evaluate the efficacy of Somah in extended extracorporeal static storage of DCD kidneys using insights gleaned from our experience of preservation of other organs in Somah and studies published in the field, as a standard for preservation of renal grafts cellular viability and energy requirements during storage.
Female Yorkshire Swine weighing 40-50 Kgs were used as per protocol approved by institutional animal studies committee. Swine were sedated with telazol 4-6 mg/kg i.m. and xylazine 2 mg/kg i.m., intubated and connected to ventilator. Anesthesia was maintained using i.v. propofol (10 mg/kg/hr) and remifentanyl (40-60 μg/hr). Cis-atracurium (10-20 mg i.v.), a paralytic agent, was administered ten minutes prior to surgery. Upon midline sternotomy, animals were systemically heparinized (300 mg/Kg) and aortic root cannulated. Ice cold cardioplegia (20 mM K+) was infused after aortic clamping to stop the heart which was then excised for other experiments as described [6,7]. Time of complete cessation of heart contraction was recorded as the beginning of warm ischemia of other body organs. Post median laparotomy, suprahepatic aorta was cannulated and abdominal organs flushed with 2 L of ice cold UW (CoStorSol; Preservation Solutions Inc., Elkhorn, WI) or Somah solution (Somahlution Inc., Jupiter, FL), at 100 mmHg pressure and a flow of 300 ml/min, till perfusate returning through inferior vena cava (IVC) was clear. Abdominal organ harvest was concluded first with hepatectomy for use in other experiments, and subsequent total bilateral nephrectomy after carefully dissecting the renal pedicles. Kidneys were immediately transferred to Somah or UW solution (Table 1) at 4°C and static stored for 72 hours. Kidney biopsies were obtained for histopathology, HEP and Western blot assays at time 0, and 6, 24 and 72 hour time-points. Time 0 corresponds to 1 hour in storage; time required to transport kidneys from animal research facility to lab before first biopsy.
Tissue was fixed in formalin and embedded in paraffin before cutting 10 μ thin sections that were melted onto glass slides for further processing. Tissue sections were dried in sequentially increasing ethanol concentrations and then subjected to hematoxylin and eosin staining after which slides were immersed in xylazine clearing agent, covered with cover slip and examined under microscope. Images were acquired and analyzed using Olympus microscope and image analyzer system (BX51TRF; Olympus America Inc, USA) and assessed in blind fashion by independent observers.
ATP and creatine phosphate (CP) were measured in kidney tissue extracts as described [4-7,10]. In brief, 20 mg of renal tissue was suspended in 400 μl of 0.4 M ice-cold perchloric acid and homogenized twice for 30 secs. Homogenate was centrifuged at 1970 g for 10 mins at 0°C. An aliquot of supernatant was neutralized with equal volume of icecold 0.4 M KHCO3 solution and centrifuged as above. Supernatant was stored at -80°C for ATP/CP measurements. The pellet was dissolved in equal volume of 0.1 M NaOH and centrifuged and used for protein assay. ATP/CP was measured using a bioluminescent assay kit (Sigma- Aldrich and GloMax Multi+Detection System, Promega) according to manufacturer’s protocol.

Table 1: Composition of Somah and UW solutions
Protein extraction: 20 mg of kidney tissue was suspended in extraction buffer containing a protease inhibitor cocktail. Tissue was homogenized for 30 secs, centrifuged at 16,100 × g for 10 mins and supernatant was collected. Equal amounts of total protein (30 μg) from different samples were mixed with Laemmli sample buffer containing 5% β-Mercaptoethanol, and heated at 100°C for 3 mins.
Electrophoresis: Proteins were resolved on 10% SDS-PAGE, and electro-blotted onto nitrocellulose membrane; proteins were identified using antibodies (anti-Caveolin, eNOS, vWF, and EPO) and chemiluminescence assays and band densities were normalized to betaactin as described [4].
Changes in pH and lactate, glucose metabolism, oxygen and carbondioxide concentrations (pO2 and pCO2) in Somah and UW were assessed at 0 (1 hour; vide supra), 6, 24 and 72-hour time- points during storage using VetScan iStat and VetScan VS2.
The measurements and data extraction were performed in blinded fashion. Comparison within two groups (UW vs. Somah, n=7 in each group) was conducted to evaluate effects of the two solutions. Quantified initial values of various assays were compared to subsequent time points within each group using one-way analysis-of-variance (ANOVA), followed by Dunnett’s multiple comparison test, and t-test for comparative analysis between groups. Statistical significance was accepted at 95% confidence level (p<0.05). All values used were mean ± SEM unless otherwise indicated. All analyses were performed using GraphPad Prism 6 (v6.1). The authors had access to and take full responsibility for integrity of data. All authors have read and agree to the manuscript as written.
Gross morphology of kidneys: Kidneys were examined during 1-3 days of immersion static storage at 4°C. Kidneys stored in UW showed dusky and mottled appearance (Figure 1a), indicative of organ congestion. In contrast, kidneys stored in Somah appeared healthy, of uniform color and morphologically unaltered after 3 days storage (Figure 1d).
Histomorphology of kidneys: Irrespective of storage solution, there was no evident interstitial edema in all DCD kidneys at observed time-points, with well preserved overall structure of renal tissue (Figures 1b,1c,1e and 1f). The normal amorphous collection in tubular lumen was observed in proximal convoluted tubules (PCT) at all time-points and not increased with storage duration. Distal convoluted tubules (DCT) remained mostly clear of any debris at all time-points except at 72 hour where a minimal to moderate epithelial denudation was apparent in both UW and Somah-preserved kidneys (Figures 1c and 1f). Renal glomeruli exhibited normal cellularity with normal appearing Bowman’s space and continuous parietal epithelium at all time-points, in both solutions (Figures 1b,1c,1e and 1f).
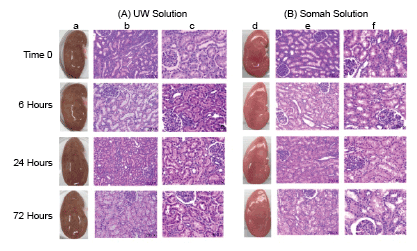
Figure 1: Porcine DCD kidneys were stored in UW (A; a,b,c) or Somah (B; d,e,f) solution for 72 hours and images were obtained for gross morphological evaluation and biopsies taken for histopathology at time 0 (see text), and 6, 24 and 72 hours of extracorporeal preservation at 4°C. Kidneys flushed with UW displayed a mottled appearance with patchy discoloration at all time points (a). Kidneys flushed with Somah displayed uniform color and smooth morphology without patchy changes (d). Histological examination showed absence of interstitial edema in both UW (b, 200X; c, 400X) and Somah (e, 200X; f, 400X) stored DCD kidneys at all the investigated time points. Higher magnifications showed a greater propensity for nuclear hyperchromacity of tubular epithelial cells of UW kidneys (c) as compared to Somah kidneys (f) at 6, 24 and 72 hour time points.
A gradual time-dependent loss of tubular epithelial nuclear heterogeneity with increased hyperchromaticity and occasional loss of cellular margins was observed in both UW and Somah-stored kidneys, indicative of tubular epithelial injury (Figures 1c and 1f). However, extent of these changes were significantly greater in UW-stored kidneys (p<0.05). On an average 3.5%, 24.4%, 39.7% and 37% in UW kidneys, and 4.4%, 6.2%, 10.9% and 11.6% in Somah kidneys, of the epithelial cell nuclei (of PCT/DCT’s) were severely hyperchromatic, at time 0, and 6, 24 and 72 hour storage time-points, respectively, suggesting that renal tubular epithelial cells in Somah-stored DCD kidneys were able to endure ischemia for a longer duration than UW-stored kidneys.
DCD kidneys were evaluated for physiological/biochemical viability by assessing metabolic functions during extracorporeal storage; demonstrating differentially active metabolism in UW and Somah groups. While freshly reconstituted solutions started off with a more alkaline pH (moreso in UW), a time-dependent fall in pH was apparent in Somah, but remained more acidic (6.8-7.2) compared to UW (7.5-7.4) (Figure 2A). Interestingly, while there was a temporal increase in glucose levels in UW solution, suggesting glycogenolysis, there was a comparative significant fall (p<0.05) in glucose levels in Somah, at and beyond 6 hour time-points, suggesting utilization of glucose present in Somah by kidney tissue/cells (Figure 2B). Inversely, compared to Somah, there was a significantly greater (p<0.05) rise in lactate levels in UW at 72 hour time-point (Figure 2C).
Since in an open experimental system as ours, the solubility of atmospheric oxygen in solution is inversely related to temperature, and since oxygen consumption follows zero-order kinetics, and as Somah and UW both were supersaturated with oxygen at 4°C, with a pO2 of 200 ± 13 mmHg at all times (Figure 2D), oxygen utilization by kidneys could not be clearly demonstrated. However, in contrast to UW, in which pCO2 was significantly lower than in Somah throughout storage (7.28 ± 0.40 mmHg at 1-hour and 7.50 ± 0.48 mmHg at 72 hours), a significant initial increase in pCO2 was observed in Somah (p<0.01) (from 5.8 ± 1.15 mmHg after initial immersion of kidney to 17.00 ± 0.45 mmHg) within 1 hour of DCD kidney storage, and remained consistently high during 72 hour period, indicating oxidative metabolic turnover right from the start of storage period (Figure 2E). It must be noted that pCO2, an indicator of dissolved CO2, does not change in Somah or UW solution in the absence of organs (not shown). Probability of bicarbonates present in Somah contributing to increase in pCO2 was negated by the fact that HCO3--concentration remained mostly unaltered (4.86 mM/L) during 72 hour storage. Conversely, HCO3-- concentration decreased from 6.30 to 4.33 mM/L in UW during 72 hour storage that may have contributed to the non-significant increase in pCO2 (Figure 2E).
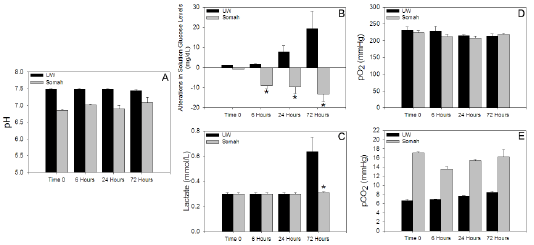
Figure 2: Graphs show the alterations in metabolic parameters in the UW or Somah solutions storing DCD kidneys over a 72-hour period. (a) pH (b) Glucose (c) Lactate (d) pO2 and (e) pCO2
The UW kidney tissue ATP, Creatine Phosphate (CP) and total HEP concentrations decreased linearly and significantly (p<0.05) during hypothermic storage. HEP dropped by 20% within six hours (Figure 3), with a net decrease of 45% at the end of 72 hour storage. In contrast, ATP, CP and total HEP levels did not change appreciably in Somah kidneys, and any decrease in ATP was compensated with a parallel increase in CP concentration, thus maintaining superior total energy levels in renal tissue during storage.
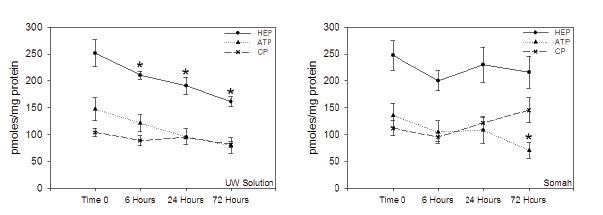
Figure 3: Alterations in energy metabolism in the UW (left) and Somah (right) stored DCD kidneys during the 72 hour extracorporeal preservation
period.
*Significantly different from Time 0 (p<0.05)
The expression of caveolin, eNOS, vWF and EPO was well preserved in DCD kidneys preserved in Somah, during the entire storage period (Figure 4). In contrast, while expression of caveolin protein was unaltered in UW-preserved kidneys as well, there was time-dependent decrease in expression of eNOS, vWF and EPO, indicative of possible renal tissue damage.
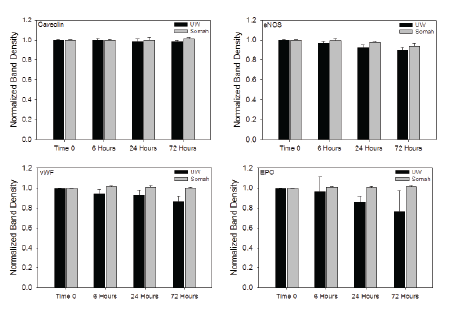
Figure 4: Graphs show time-dependent alterations in expression of caveolin, endothelial nitric oxide synthase (eNOS), von-Willebrands factor (vWF) and erythropoietin (EPO) proteins in DCD kidneys stored in either UW or Somah solution for 72 hours.
Being one of the most resistant internal organs to ischemia, use of kidneys from deceased (DCD) donors for transplantation has been commonly practiced worldwide [11-13]. While DCD pool of kidneys is still highly underutilized, prognosis of DCD kidneys upon transplantation is associated with an observably increased incidence of DGF, PNF and lowered graft life expectancy in recipients. The results of our present study suggest that by maintaining energy levels in DCD kidneys during storage using Somah, subtle damage at cellular levels can be avoided; thus possible avenues to enhance DCD kidney storage and post-transplant prognosis could be uncovered.
Our results show that UW-preserved kidneys display patchy areas of discoloration within a few minutes of organ perfusion with UW, upon harvesting, and did not resolve during 72 hour storage. In contrast, Somah-preserved kidneys remained uniform in their external morphology at all time-points. While Somah has a viscosity close to that of normal saline, UW is characterized by a much higher viscosity due to hydroxyl-ethyl starch (HES; Table 1) [14,15]. While HES helps to prevent organ edema during storage, it increases solution density that can interfere with perfusion of all parts of the organ. Although gross morphology is not the best indicator of organ viability, it is probable that an uneven perfusion of kidneys during harvesting, despite copious use of solution for perfusion, could have resulted in patchy discoloration of kidneys, due to greater UW viscosity (Figure 1).
Remarkably, renal histopathology showed no gross ultrastructural changes in either cortical or medullary regions of UW or Somahstored kidneys. However, higher magnifications revealed subtle changes in cellular nuclei, especially in tubular epithelial cells, characterized by loss of nuclear heterochromacity with increased hyperchromacity, significantly greater in UW-stored kidneys. This is consistent with an inadequacy of UW to reach all parts of kidneys (vide supra), while Somah reached effectively and provided necessary nutrients to tissues in their entirety during harvesting and extracorporeal storage, thus avoiding development of minor lesions and may potentially improve the post-transplant outcomes. Interestingly, despite better endurance of renal tissue in Somah, Somah pH was more acidotic, compared to UW, at all time-points. While acidic pH is reported to be constitutively beneficial to hepatocytes, sinusoidal epithelial cells as well as cardiomyocytes [16], to best of our knowledge, this is the first report showing advantage of a relatively acidic environment in extracorporeal kidney preservation.
Interestingly, glucose, an important energy source for highly metabolic renal tissue, was excluded from kidney preservation solutions used in current practice as it was thought to cause edema by enhanced lactate accumulation, a product of anaerobic metabolism during extracorporeal storage [17,18]. UW, a commonly used solution for renal storage, does not contain any glucose either. However, that glucose is an important energy source during extracorporeal renal storage was demonstrated by an endogenous increase in glucose levels in UW, likely due to renal glycogenolysis, and a corresponding fall in glucose levels in Somah, which innately contains high concentration of glucose (Table 1). However, even after prolonged storage of kidneys in Somah, we did not observe development of edema by gross or histological examination, contrary to previous studies involving other solutions [17,18].
After 72 hour kidney storage, UW demonstrated substantial rise in deleterious lactate levels that remained low at all time-points in Somah (Figure 2C), despite metabolism (anaerobic and aerobic) of glucose, leading to corresponding maintenance of tissue HEP in Somah kidneys (Figure 3). Aerobic oxidative phosphorylation activity in Somah kidneys was confirmed by observed increase in metabolic CO2 in Somah during storage. This was an expected result; as Somah also contains dichloroacetate (DCA), a compound that increases the activity of pyruvate dehydrogenase complex thus enhancing conversion of pyruvate to acetyl-CoA, preventing accumulation of lactate [19]; confirming our observations in hearts and livers stored in Somah [4- 8]. Furthermore, DCA, by virtue of its vessel-preservation abilities, may help prevent development of post-transplant renal artery stenosis, and further improve prognosis of transplanted DCD kidneys [20].
A significant loss of energy state (HEP) in explanted organs during storage leads to irreversible degenerative changes in the organ [21]. It is thus imperative for preservative solution to maintain organ in homeostasis and/or allow it to recover during extended storage by modulating organs metabolic pathways. Despite potential glycogenolysis-dependent increase in glucose concentration in UW during storage (Figure 2B), a significant depletion of renal HEP stores was apparent, in contrast to obvious preservation of energy state in Somah kidneys (Figure 3). This suggests that UW kidneys were highly catabolic, leading to loss of HEP’s and tissue injury, (Figure 1). In contrast, greater oxidative phosphorylation of glucose in Somah-stored kidneys, facilitates greater HEP generation (for equivalent glucose molecules), than by anaerobic glycolysis alone [22] thus enhancing the organs energy state during storage. The low HEP levels observed in DCD UW-kidneys, could predictably result in delayed graft function (DGF) at the least, and even primary non-function (PNF). Therefore, despite the predominant use as preservation solution for explanted kidneys, UW may not provide optimal conditions for extended storage of DCD (or BHD) kidneys. In contrast, preservation in Somah may provide a viable alternative.
The blood vessels (capillaries) form the bulk of renal cortical tissue while tubular structures predominate in renal medulla. While glomerular tuft collapse within 6 hour storage in Histidine-Tryptophan-Ketoglutarate (HTK) solution has been reported [18], we did not find such drastic glomerular change in either Somah or UW stored kidneys, at any timepoint (Figure 1). However, a steady decline in expression of eNOS (important in vasomotor function), von- Willebrands factor (vWF; marker for blood vessel endothelium) and erythropoietin (EPO; marker of specialized peritubular epithelial cells), during 72 hour DCD kidney storage in UW, suggests subtle damage to both vascular [23] and tubular structures [24] (Figure 4). This is consistent with our histological findings of increase in tubular nuclear hyperchromacity observed in UWstored kidneys. In contrast, the expression of all investigated proteins including caveolin, eNOS, vWF and EPO were unaltered during the same periods of observation in Somah-preserved kidneys suggesting both cortical and tubular renal tissue preservation.
With the rising number of patients requiring renal transplantation, it has become extremely important to enhance availability and quality of DCD kidneys for transplant. While traditional preservation techniques are still associated with a major post-transplant inadequacy of DCD organ function, we provide preliminary evidence that use of Somah for static preservation of DCD kidneys may potentially decrease the incidence of DGF, PNF and improve graft life upon transplantation. Further studies involving different storage and perfusion conditions including renal transplantation are required for both BHD and DCD Somah preserved kidneys before our laboratory observations become a clinical reality.
We gratefully acknowledge the assistance of Diane Ghera, BS, and her staff with animal husbandry and preparation of animals for surgery. We thank Peter Hirsch, MS, for his assistance during pre and post surgery. We would like to thank Aditi Thatte for her encouragement and support, and Dr. Frances Achee and Michael Charpak for administrative support.
Download Provisional PDF Here
Article Type: Research Article
Citation: Cao H, Lowalekar SK, Lu XG, Treanor PR, Thatte HS (2016) Biochemical Evaluation of Renal Grafts Procured after Cardiac Death and Stored in Novel Solution Somah: A Pre-Clinical Study. Transplant Res J 1(1): doi http://dx.doi. org/10.16966/trj.102
Copyright: © 2016 Cao H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access