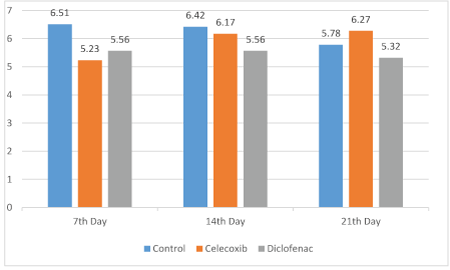
Figure 1: The number of osteoblasts in the three groups.
Samira Hajisadeghi1 Farimah Sardari2 Mostafa Sadeghi3 Sayyed Mohammad Razavi4 Michael W Roberts5*
1Assistant Professor, Dental and Oral Research Center, Department of Oral and Maxillofacial Medicine, School of Dentistry, Qom University of Medical Sciences, Qom, Iran*Corresponding author: Michael W Roberts, Professor, Department of Pediatric Dentistry, University of North Carolina School of Dentistry, Chapel Hill, North Carolina, USA, Tel: (919)537-3781; E-mail: Mike_Roberts@unc.edu
Introduction: There is extensive evidence in the orthopedic literature that prolonged use of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) can hinder long bone fracture healing and new bone formation around implants. The aim of this study was to investigate if short time administrations of NSAIDs such as diclofenac or celecoxib interfere with the course of healing of alveolar bony socket following tooth extraction.
Materials and Methods: Forty-five rats (15 per group) were used. After extraction of the right maxillary first molar, 15 rats received 5 mg/kg/day of diclofenac, 15 rats received 15 mg/kg/day of celecoxib and 15 rats received normal saline. The animals were sacrificed at 7, 14 and 21 days following tooth extraction. The number of osteoclasts, osteoblast and new bone formation were determined by using histological analyses. Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test (α=0.05).
Results: On the 7th day, the osteoblast number in the control group was higher than the diclofenac and celecoxib groups. Tartrate-Resistant Acid Phosphatase (TRAP) immunolabeling of the control group was more than the diclofenac group on the 7th day and more than the celecoxib group on the 14th day. On the 21st day, no significant differences were noted among the three studied groups. On the 14th and 21st day, new bone formation in the NSAID treated rats was not significantly different from that in the control rats.
Conclusion: Short-term treatment with diclofenac or celecoxib, although they have the capacity to inhibit the enzyme cyclooxygenase, did not cause a significant delay in alveolar bone healing during the experimental period in rats.
Anti-inflammatory agents; Celecoxib; Diclofenac; NSAIDs; Alveolar bone; Healing
The conversion of arachidonic acid to prostaglandins was catalyzed by the cyclooxygenase enzymes COX-1 and COX-2, which modulate osteoblastic and osteoclastic functions and exert an anabolic effect on bone in the healing process [1]. The nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit COX-1 and COX-2 activity and, consequently, prostaglandins synthesis [2]. NSAIDs are frequently used in the control of fever and pain, edema, in acute post-surgical or posttraumatic pain, and for relief of chronic pain associated with muscleskeletal disorders. NSAIDs are commonly used drugs for management of pain after tooth extraction [3]. The NSAIDs may delay the repair of damaged tissues, such as the fractured bone, cartilage, skin and new bone formation after tooth extraction [4,5].
It has been shown in rodent studies that NSAIDs may disturb not only bone healing but also normal bone growth [6,7]. Despite the unequivocal evidence that NSAIDs may delay recovery of damaged tissues, the precise mechanism of this effect is not fully understood. Currently, numerous NSAIDs are available, from the conventional nonselective NSAIDs which inhibit non-selectively both COX-1/ COX-2 enzymes, toward a new class of highly selective COX-2 inhibitors, the coxibs. Diclofenac (inhibits non-selectively both COX1 and COX-2 enzymes) and celecoxib (selectively inhibits COX-2) are NSAIDs presenting significant analgesic and anti-inflammatory activities [3,8].
In orthopedics, prolonged use of both nonselective and selective NSAIDs can hinder reparation long bone formation [8]. Even though, the conventional NSAIDs are commonly used in dentistry for pain and swelling management, very few assessments have been conducted to assess their deleterious effects on healing of the alveolar bony socket [9].
There are only limited data on the effects of NSAIDs on wound healing after oral surgery. The present study was designed to investigate whether diclofenac and celecoxib have a detrimental effect on alveolar bone healing, taking the filling of extraction socket with new formed bone as an experimental model of alveolar bone formation.
Forty five male Wistar rats at 8-10 weeks of age, weighing 200 ± 25 g were used in this study. The animals were housed under similar conditions (22°C room temperature, 40% humidity and 12 hours light/12 hours dark cycle, with free access to water and rat chow) during the study period. This study was assessed and approved by Animal Research Ethics Committee of the Isfahan University of Medical Sciences, Iran and the principles of laboratory animal care and national laws on animal use were followed in the present study [10].
General anesthesia was induced by intramuscular injection of ketamine 10% (Alfasan International, Woerden, Holland, 80 mg/kg) and Xylasine (Neurotranq, Alfasan, Woerden, Holland, 8 mg/kg) in all rats and their right upper molars were luxated with the aid of a tapered instrument and extracted with a modified hemostat (two cavities were made in each beak). An atraumatic surgical technique was used to extract the teeth. The animals were observed until fully recovered; no antibiotic or other medication was used.
The animals were randomly assigned into three groups. The celecoxib group received a daily dose (15 mg/kg body weight of celecoxib (Daroupakhsh Co, Tehran, Iran) by gavage administration; the diclofenac group received a daily dose (5 mg/kg body weight of diclofenac sodium (Daroupakhsh Co) diluted with sterile distilled water and was injected subcutaneously. The control group received only normal saline, daily. Medications in all groups were administered for a period of seven days, starting on the day of tooth extraction. Doses of all drugs were chosen based on prior studies and pharmacokinetic data to mimic similar doses used to treat humans, taking into account the differences in metabolism between species [3,11,12].
Five animals from each group were sacrificed on the 7th, 14th and 21st day post extraction by an over dose of ether inhalation. The mandibles was removed and the maxillas were fixed in 4% paraformaldehyde solution (SRPC, Tehran, Iran), demineralized with 10% Ethylenediaminetetraacetic acid (EDTA) (Merck, Darmstadt, Germany) and embedded in paraffin (Merck). The pieces were sectioned perpendicular to the long axis of the alveolar process with a microtome (Accu-Cut SRM, SAKURA, USA) in order to obtain slices of five µm thicknesses, which were mounted in previously poly-Llysine slides.
A slide using Hematoxylin-Eosin (H&E) stain was prepared from each specimen. Sections representative of the number of osteoblast and amount of new bone formation in each tooth were captured by a digital camera (Axio Cam MRc5; Carl Zeiss do Brasil Ltda, Rio de Janeiro, RJ, Brazil) coupled to a light microscope (Olympus CX21FS, Olympus Corporation, Tokyo, Japan) with 1:100 magnification. For the immunohistochemistry reactions, blocking with 0.03% hydrogen peroxide followed by primary antibodies anti Tartrate-Resistant Acid Phosphatase (TRAP) (Goat anti trap polyclonal-Santa Cruz, CA, USA) and the biotinylated donkey anti-goat antibodies (BiotinSP-AffiniPure donkey anti-goat IgG-Jackson Immunoresearch Laboratories, West Grove, PA, USA) was the secondary antibody; the immunohistochemistry reaction signal was amplified with the Avidin-Biotin system (Kit ABC Vectastain Elite ABC, Vector Laboratories, Burlingame, CA, USA) and the reaction was revealed using diaminobenzidine (DAB-Sigma, Saint Louis, MO, USA) as the chromogen.
Additional sections were used for immunohistochemical staining to determine the expression of TRAP protein in the alveolar tissues during the healing process from tooth extraction, and then were counterstained with Harris’s hematoxylin. Immunostaining was used to evaluate alveolar bone under conventional optical microscope. A negative control was prepared for each specimen using the same method except for the primary antibody. Sections representative of TRAP protein in each tooth were captured by a digital camera (Axio Cam MRc5; Carl Zeiss do Brasil Ltda., Rio de Janeiro, RJ, Brazil) coupled to a light microscope (Olympus CX21FS, Olympus Corporation, Tokyo, Japan) with 1:400 magnification. Slides were examined by an expert blinded pathologist.
The results are presented as means ± SD, and statistical differences among studied groups were assessed by one-way ANOVA followed by Tukey’s post hoc test. All analyses were done using SPSS-20 (SPSS Inc., Chicago, IL, USA) and P value less than 0.05 were considered significant.
Throughout the study period, all animals tolerated the experiment. Behavior and body weight among the groups were not significantly different (P>0.05).
The results of the comparison of osteoblast number, TRAP (osteoclast number) and new bone formation in the dental socket among the groups are shown in figures 1 and 2. As shown, the number of osteoblast on the 7th day in the control group was significantly greater than the diclofenac and celecoxib groups (P<0.05); but at 14th and 21st day, differences among the groups were not statistically different (P>0.05) (Figure 1). The number of TRAP-positive osteoclasts among the groups at 7 and 14 days were statistically significant (P<0.05). The control number of TRAP was significantly higher than the diclofenac and the celecoxib groups at 14 days (P<0.05). Although, the number of osteoclasts observed in the control group was greater compared with diclofenac and celecoxib groups on the 21st day, the differences were not statistically significant (P>0.05) (Figure 3). New bone formation at 7 days among the studied groups was significantly different. The new bone growth in the diclofenac and celecoxib groups was significantly less than the control group at 7 days (P<0.05), but the difference between the diclofenac and celecoxib groups was not significant (P>0.05). On the 14th and 21st day, new bone formation among studied groups was similar and there were no statistical significant among groups (P>0.05) (Figures 3 and 4).

Figure 1: The number of osteoblasts in the three groups.
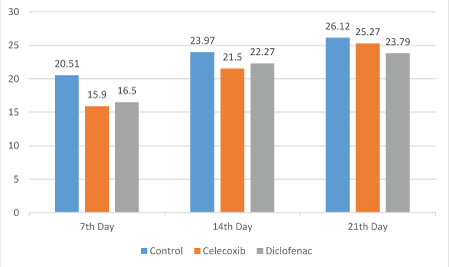
Figure 2: New bone formation in the three groups.
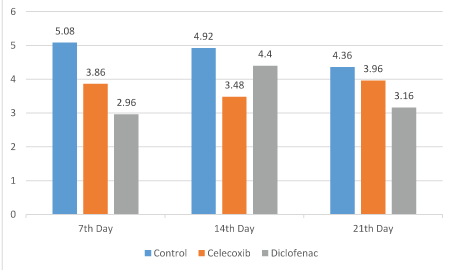
Figure 3: The number of TRAP-positive osteoclasts in the three groups.
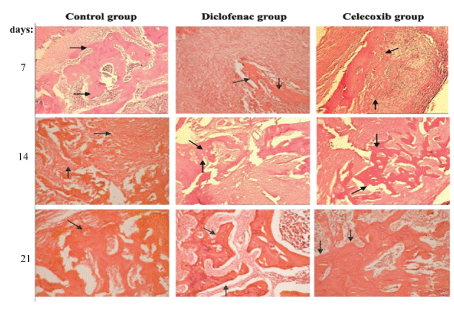
Figure 4: New bone formation at 7th, 14th and 21st post-extraction days in the control, diclofenac and celecoxib groups.
A suitable model for the study of bone formation is provided for alveolar healing and can be considered a sensitive indicator of bone damage and repair [13,14]. In different animal species, the process of intra-alveolar healing is well established [15,16]. In brief, after a tooth is extracted the socket is filled with a blood clot which is progressively invaded by capillary sprouting and fibroblasts originating from periodontal ligament remnants. In the sequence, the amount of inflammatory cells and blood vessels decreases, the osteoblasts become evident, and the coagulum is gradually absorbed and replaced by immature connective tissue. An immature bone matrix initially synthesize by osteoblasts that is further mineralized by calcium deposition as hydroxyapatite crystals. The alveolar bone neoformation takes place toward the center from the lateral and apical walls, and the healing process culminates with filling of the dental socket by trabecular bone [17,18].
In this study, the histological feature of the alveolar socket healing was observed in a control and animals treated with two different types of NSAIDs (diclofenac and celecoxib) and followed for 21 days. It has been shown in previous rat studies that the maximum mineral bone density and the major proportion of bone formation takes place by the end of the second week after tooth extraction [19,20]. In this study the hypothesis that NSAIDs could hinder the alveolar bone healing was tested. Our results showed that in the first week osteoblast, TRAPpositive osteoclasts and new bone formation of diclofenac and celecoxib groups were lower than the control group. The differences between the control group and the study groups in osteoblast, osteoclast and bone formation was significant in the first week, there was no significant difference between the three groups in these criteria by the end of the third week. It would appear that the short-time administration of a low dose of NSAID for pain relief after tooth extraction may cause initial delay in healing, but no important negative effect occurred in long term bone healing. In vivo studies found similar impairments of fracture healing by NSAIDs, primarily in the early phase of healing [21,22].
Osteoclasts are distinguished from macrophages by the presence of TRAP in their cytoplasm. This type-V isoenzyme of acid phosphatase presents an intense activity in osteoclasts, being considered a specific marker for osteoclasts. Histological analysis is an effective method to identifying osteoclasts and analyzing their dynamics as is staining of TRAP, a cytochemical marker for osteoclasts [23]. In our study, diclofenac sodium had lower clastic activity, as demonstrated by lower TRAP expression. This drug also led to a decrease in TRAP expression three weeks post extraction; especially in the first seven days. Histologically observations also confirmed the presence of clastic cells. Celecoxib led to lower clastic activity, especially in the second week. Other studies have shown that celecoxib and diclofenac reduced osteoclastogenesis in vitro [11,24,25]. They have also shown that NSAIDs inhibit the number of osteoblast in rats at a very early stage [26,27].
A nonselective inhibition of the enzymes COX-1 and COX-2 by conventional NSAIDs have demonstrated interference with long bone fracture healing and rate of spinal fusion but an absence of this deleterious effect has also been observed. The effects of selective COX-2 inhibition by NSAIDs were evaluated in several studies but the results were equivocal even though the importance of COX-2 and prostaglandin E2 for bone formation is well known [8].
The effect of COX inhibition on alveolar bone healing is minimal. It has also been reported that short-term treatment (four days) with diclofenac in rats delayed alveolar socket healing [28], while other studies showed that NSAIDs didn’t hinder alveolar bone healing [27]. There are few studies that have used NSAIDs to control edema and pain after removal of impacted third molars and often refer to shortterm drug administration. Some authors have reported no significant difference in the rate of bone healing following administration of aspirin, diclofenac, ibuprofen, and flurbiprofen [9,29,30].
The hypothetic disadvantageous effect of diclofenac and celecoxib on the healing of alveolar bony socket was not established in the present study. Some factors may explain the disagreements in the experimental outcomes about the effects of COX inhibitors on bone healing, such as duration of treatment and dosage, as well as age, intra and inter species differences regarding sensitivity to drugs, pharmacokinetics of drugs, compensatory local and systemic factors, and rate of bone remodeling [31,32].
The present results provide evidence that diclofenac and celecoxib do not interfere significantly with alveolar bone healing. Also, additional studies in a diversity of experimental models to definitely confirm, or discharge, the lack of interference of this drug with new bone formation with respect to duration of treatment and dosage is indicated.
We thank to the Department of Oral and Maxillofacial Medicine and Torabinejad Dental Research Center, School of Dentistry, Isfahan University of Medical Sciences, Isfahan for financial support of this study
The authors have no financial or personal relationship with other people or organizations that could inappropriately influence or bias this paper.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Hajisadeghi S, Sardari F, Sadeghi M, Razavi SM, Roberts MW (2018) Comparison of the Effects of Diclofenac Sodium or Celecoxib on the Healing Tooth Socket. A Study in Rats. J Surg Open Access 4(2): dx.doi.org/10.16966/2470-0991.170
Copyright: © 2018 Hajisadeghi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access