Figure 1: Motiva Implants® with Q Inside Technology.
Michael T Nelson1 Kurt A Brattain2 Jeffrey M Williams3*
1Professor of Radiology, University of Minnesota, Minneapolis, Minnesota and CEO, Breast-Med, Inc., Minneapolis, Minnesota, USA*Corresponding author: Jeffrey M Williams, Geissler Companies, 14505 21st Avenue North, Minneapolis, MN 55447. Tel: +1.763.550.9400, Fax: +1.763.550.1152, E-mail: jwilliams@geisslercorp.com
Life-Cycle traceability for medical devices is critical for assurance of patient safety and of great concern for manufacturers, healthcare providers and global regulatory authorities. Electronic tracking technologies are relied upon to maintain traceability integrity throughout the supply chain until device use, or in the case of implantable medical devices, placement in the patient. Active implantable medical devices, such as cardiac pacemakers can be identified in vivo but passive medical device traceability post-implantation must rely on patient registration cards and patient history records. Motiva Implants® with Q Inside Safety Technology™ silicone gel-filled breast implants contain a radiofrequency identification device and are the first passive devices that can be identified in vivo, but its presence creates an artifact during MRI, raising the concern of possibly missing a cancer diagnosis during surveillance of high-risk patients. Dual-modality imaging, using MRI and ultrasonography when the artifact is present is essentially equivalent to MRI alone when the artifact is not present, based on a number of potentially missed cancer detections per 1,000 screening exams. The Number Needed to Harm (NNH) with MRI with artifact present and obstructing 5.37% of the breast implant image indicates one high-risk patient with a cancer reoccurrence would likely be missed for every 596 high-risk patient screening exams performed. Likewise, when dual modality of MRI and ultrasonography are used to study the high-risk patient group, it would take 17,892 screening exams before a patient with cancer recurrence is likely to be missed (false negative). The addition of ultrasonography to the artifact void area mitigates the impact of the artifact quite substantially. Concerning traceability, the ratio of electronic in vivo assures a 100% traceability benefit (high-risk patients with cancer not missed in imaging studies) to the harm caused by the artifact (high-risk patients with cancer missed in imaging studies). Even for the MRI study alone with the artifact, 100% traceability finds a 22.84-fold increase in the number of patients benefiting over the number of patients harmed. Dual-modality improves this ratio up to a 710.96-fold increase of the number of patients benefiting over the number of patients harmed.
Breast Implants (AE); Magnetic Resonance Imaging (SN); Artifacts; Ultrasonography; Mammary (SN); Risk Assessment
RFID-M: Radiofrequency Identification Device Micro Transponder; ESN: Electronic Serial Number; FDA: United States Food & Drug Administration; PIP: Poly Implant Prostheses; ALCL: Anaplastic Large Cell Lymphoma; ASIC: Application Specific Integrated Circuit; MRI: Magnetic Resonance Imaging; EC: European Commission; GUDID: Global Unique Device Identifier Database; NNH: Number Needed To Harm
The purpose of this paper is to discuss the safety of in vivo electronic identification enablement in implantable silicone gel-filled breast implants and to present an approach to cancer surveillance in a high-risk patient that appears to minimize the risk of failure to diagnose cancer occurrence/reoccurrence. A risk impact analysis is presented that demonstrates the promise of dual-modality imaging in a high-risk patient cohort when an artifact is present in the imaging area.
Implantable radio-frequency identification devices micro transponders (RFID-M) are passive devices that emit an electronic 15-digit electronic serial number (ESN) when interrogated by a hand-held reader tuned to the same RF, typically between 128 KHz and 135 KHz. The first in vivo applications of RFID technology began approximately 30 years ago with implementation in larger, higher value animals wherein the incremental costs of RFID technology were justifiable. In the equine markets, horse identification by physical description, iron brand, and tattoos were usually, but not always, adequate. Identity manipulation and fraud were also possible. The security of assuring pedigree confidence, preventing animal substitution in racing, sales and exhibition, preventing thievery and fraud prevention, and tracking expensive animals during transportation by third-party handlers quickly drove adoption of RFID-M identification [1]. RFID-M implantation in the veterinary markets grew quickly, with 8.2 million dogs receiving RFID-M implants by 2005 [2].
Clinical adoption of implantable RFID-M technology is largely driven by the criticality of medical product traceability. Supply chain traceability is well developed and highly accurate, largely due to electronic tracking technology [3]. After an implantable medical device is placed in a patient traceability is dependent on identification cards are given to the patient or some other type of human intervention.
Accurate, life-cycle medical device traceability is an issue of high concern for regulatory authorities, manufacturers, healthcare providers, and patients. RFID-M implementation for assurance of accurate traceability, and when referenced back to either manufacturing or patient history records, can identify the manufacturer, brand, and model. Each RFID-M has a unique, retrievable ESN that can be verified throughout the life-cycle of a medical device with the RFID-M onboard.
Establishment Labs, S.A. (Alajuela, Costa Rica) a subsidiary of Establishment Labs Holdings Inc (New York, USA) is the first medical device manufacturer to incorporate an RFID-M into an implantable medical device. They market Motiva Implants® with Q INSIDE SAFETY TECHNOLOGY™ silicone gel-filled breast implants that have an embedded RFID-M and a proprietary, hand-held RFID-M reader. Immediate, accurate, in vivo product recognition, is a benefit that translates to improved safety for the patient.
Devices of this type have been used in the veterinary world for approximately 30 years, their use has just recently been adopted for human use. While the benefit may seem obvious, this report discusses the risks that may be introduced when an RFID-M is embedded in a silicone gel-filled breast implant.
The RFID-M used for the “Q with Safety Inside”, a small glass-encased RFID-M, measuring 2.1 mm x 9 mm, has been cleared by the U.S. Food & Drug Administration (FDA) for use in humans and is available in CE-marked Motiva Implants®. (Figure 1). The RFID-M is manufactured by JAMM Technologies (Minneapolis, USA) and the RFID-M reader is manufacturer is iD Porte, Ltd. (Guernsey, Channel Islands, U.K.) (Figure 2).

Figure 1: Motiva Implants® with Q Inside Technology.
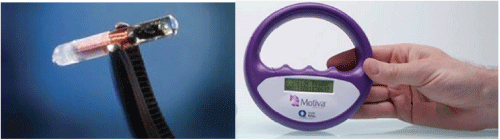
Figure 2: Motiva Implants® with Safety Inside Technology RFID-M and Motiva® Reader.
RFID-M Enablement of Silicone Gel-Filled Implants: Patient Safety Considerations
To understand how RFID-M embedment in a silicone gelfilled breast implant improves safety for the patient, consider the debacle that occurred when Poly Implant Prostheses, (PIP) manufactured and sold silicone gel-filled breast implants filled with unapproved, industrial-grade silicone that did not comply with CE marking, and a flawed shell structure, leading to high rates of implant failure [4]. Regulatory authorities forced the company to remove the PIP implants from the market, but not before approximately 40,000 women had received PIP implants. The majority of PIP implants were fitted in private clinics, but a small number were United Kingdom National Health Service patients, mostly for breast reconstruction after breast cancer [5]. The PIP scandal created a serious concern among surgeons and patients that could only be addressed if the surgeon or patient was able to retrieve the implant record or patient registration card. Lacking implant information regarding brand, lot and manufacturing date, some surgeons recommended elective explant, thereby submitting the patient to surgical risk, mental duress, and significant expense. Women with PIP implants described harrowing experiences, reduced quality of life, and anxiety related to implant risks and uncertainty regarding appropriate clinical actions [6]. Physicians who trusted the product and regulatory agencies were unaware of the defects of the implants and inadvertently exposed their patients to increased adverse risks. The damage to the reputations of these physicians’ practices and institutions has been considerable [4].
Another potential safety benefit relates to the growing concern about the causative effect of silicone gel-filled breast implants in anaplastic large cell lymphoma (ALCL) [7]. A review of governmental authority databases published in 2017 indicated that ALCL is more prevalent with textured surface breast implants than with smooth surface breast implants (50% vs. 4.2%, p=0.0001) [8]. Thus, as in the PIP debacle discussed above, there is a critical need for the healthcare provider and patient to know what brand and type of breast implant are now and as time continues.
But RFID-M can increase the risk for the patient. As is true with any foreign body, RFID-M causes an imaging void artifact to be present during imaging sequences. Table 1 provides a general description of the RFID-M artifact during imaging with different modalities.
| Imaging Compatibility | Appearance |
| MRI | Void artifact 20-30 mm radially from RFID-M |
| Ultrasound | Good echogenicity |
| Digital X-ray | Sharp border attenuation consistent with metal material |
| Tomosynthesis | Sharp border attenuation consistent with metal material |
| C-View Tomosynthesis | Sharp border attenuation consistent with metal material |
| Faxitron | Good Visualization of RFID-M |
Table 1: RFID-M Imaging Characteristics during Different Imaging Modalities.
The RFID-M comprises an application specific integrated circuit (ASIC), and a ferrite core/copper antenna, contained within a sealed biocompatible glass tube (2 mm × 9 mm). Imaging voids or artifacts are common when foreign bodies are present [9]. The imaging void created by the RFID-M during magnetic resonance imaging (MRI) is larger than the device itself, approximating 20 mm to 30 mm. This imaging void is near the base of the implant and will occlude a small area of the patient’s tissue nearest to the RFID-M [10].
MRI is the recommended imaging modality for scans of silicone gel-filled breast implant patients [11]. The imaging artifact represents a safety issue for the patient. Incomplete visualization of the entire scanned field could prevent the diagnosis of a new or reoccurring lesion. Artifacts may be caused by a variety of phenomena such as the underlying physics of the energy-tissue interaction (e.g., ultrasound air bubbles), data acquisition errors (e.g., patient motion), poor reconstructive algorithms (unable to properly represent the anatomy) or the presence of metallic objects (orthopedic hardware, pacemakers). Artifacts may present as shadows, distortions or create a void within the imaging field [12].
Silicone gel-filled breast implants commonly interfere with diagnostic and screening imaging examinations of the breast by compressing and distorting the breast and nearby tissues. Silicone gel-filled breast implants create shadows or voids that obscure some breast tissue. Use of a properly functioning highfield strength MRI system, a dedicated bilateral breast coil, and an optimal imaging protocol will usually provide a high-quality breast MRI [13].
An example of the RFID-M artifact is presented below. This scan demonstration was performed on a 1.5-Tesla Siemens Aera® (Siemens, A.G., Munich, Germany) using an RFID-M placed in a phantom model setup (Figure 3).
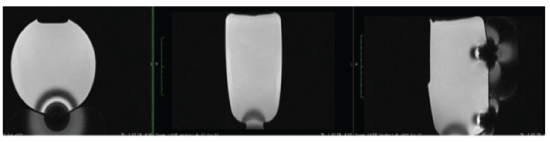
Figure 3: RFID-M Artifact during MRI in a Phantom Silicone Gel containing an RFID.
Radiologists mitigate artifact presence constantly. They have multiple strategies and tools at their disposal. Other imaging modalities such as ultrasound, tomosynthesis and digital x-ray can be used to visualize the MRI scanned voided area. There are specialized MRI scanning algorithms available to improve imaging in artifact-dense fields, e.g. when orthopedic prostheses are present in the scanning field. Use of special algorithms developed for scanning near metallic objects significantly increase scanning time and reduce artifact size by approximately 30% but do not eliminate the imaging void.
Ultrasonography efficacy is not affected by the presence of metallic bodies. The RFID-M is easily visualized with artifact creation limited to the RFID-M physical size. MRI is the optimum imaging modality for visualization of the breast implant and surrounding tissues. Ultrasound can “see” the areas that are invisible to the MRI. Implementation of a dual modality approach, MRI + ultrasound, is additive, providing complete visualization of the entire scanned area. The ultrasound image presented below was scanned using a Philips iU22 with 6 MHz – 14 MHz transducer (Koninklijke Philips N.V., Amsterdam, The Netherlands) (Figure 4).
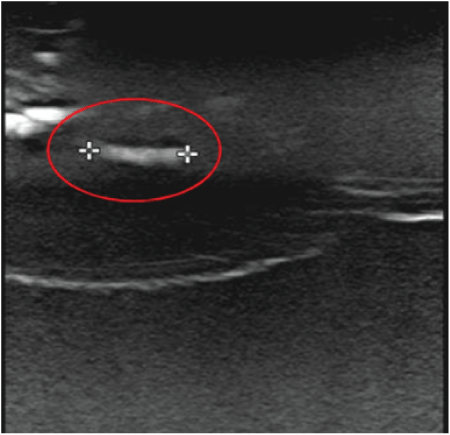
Figure 4: Ultrasound Scan (Transverse View) of RFID-M in a Phantom Model Setup.
The RFID-M manufacturer (JAMM Technologies, Minneapolis, Minnesota, USA) attempted to assess the risk of missing a cancer reoccurrence due to the presence of the RFID-M created artifact as well as the reduction of risk when a second imaging modality is included with MRI cancer surveillance screening.
Methods
Motiva Implants® with Inside Safety Technology utilization data was collected for analysis. The total number of patients and percentage of bilateral vs. unilateral placements were estimated using the manufacturer’s patient registration information. According to the manufacturer’s records, 2.5% of the breast implants in this assessment were placed for breast reconstruction; the remaining 97.5% were placed for aesthetic procedures. Adoption of RFID-M enablement of ESN identification of breast implants in vivo is of possible benefit across all patient groups, especially for patients identified as high-risk for cancer or other morbidities; in this data set, the 2.5% represents the actual high-risk patient cohort.
The rate of 2.5% of Motiva Implants® likely represents the low-end of breast implant reconstruction procedures. Other manufacturers may sell a much higher percentage of their breast implants for reconstruction procedures. For extrapolation purposes, it was decided to include a 20% estimate, including an adoption rate that is assumed to reflect an average adoption rate across all manufacturers (Table 2).
| Presence/ Absence of RFID-M | Number of Patients/Units Sold | Percentage of Total Sold | Patients Registered with manufacturer | High-Risk Cohort Based on 2.5%(Actual) | 20.0% Reconstruction Cases [Extrapolated] | 4 year Cummulative Cancer Cases Based on High-risk Cohort Count at 2.5%(Actual) | 20.0% Reconstruction cases [Extrapolated] |
| No RFID-M | 76,207 / 148,697 | 48.20% | 14.10% | 1,905 | 15,241 | 82 | 655 |
| + RFID-M | 81,940 / 159,882 | 51.80% | 21.50% | 2,049 | 16,388 | 71 | 569 |
| Total | 158,147 / 308,579 | 100.00% | 18.00% | 3,954 | 31,629 | 153 | 1224 |
Table 2: Motiva Implants® Data Set Characteristics.
The artifact area caused by the RFID-M is significant for the assessment of the risk of harm due to missing a lesion or cancer reoccurrence. For the purposes of risk probability assessment, a worst-case dimension for artifact area was assumed to be 1.5 cm radially and 5.0 cm longitudinally. The RFID-M is cylindrical, but to continue the worst-case assessment intent, a more rigorous calculation of a rectangular artifact shape was employed for the risk impact assessment. The area calculated from the dimensional estimates was calculated to be 14.98 cm2.
The distribution of Motiva Implants® sizes included in the data set is presented in figure 4, with calculations presented in table 3. The area of the breast implant affected by the artifact is inversely proportional to the implant size. The percentage of area voided by the artifact ranges from 2.77% in the largest implants to 10.28% in the smallest implant. The mean area affected for all breast implants in the data set is 5.37% (5.37% - 5.38%, 95% C.I.; 25% quartile = 4.76%, 75% quartile = 5.88%).Since the breast implant sizes (by % of data set) were mostly in the mid-size range, the voided area size is tightly grouped around the mean artifact (5.37%), thus, the mean artifact imaging void of 5.37% was used for all calculations and estimates in this assessment (Figure 5).
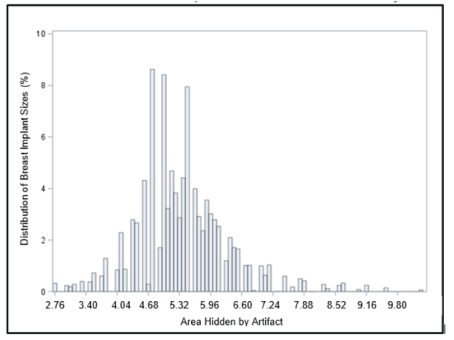
Figure 5: Distribution of Motiva Implants® with Associated Areas Voided by Artifact.
| Total Number of Motiva Units in Data Set | 308,579 |
| Artifact Area | 14.98 cm2 |
| Mean % of Artifact area size of Implant area size | 5.37% |
| Maximum % of Artifact area size of Implant area size | 10.28% |
| Minimum % of Artifact area size of Implant area size | 2.77% |
| 25th & 75th Percentile of Artifact area size of Implant area size | 4.76% - 5.88% |
| 95% Confidence Interval of Artifact area size of Implant area size | 5.37% - 5.38% |
Table 3: Artifact Area Calculations and Assumptions.
To evaluate the potential benefit of adding ultrasound as a second imaging modality with MRI, risk impact analyses were conducted on MRI, ultrasound, and MRI + ultrasound and the models were evaluated in the presence of, or absence of, artifact. Commonly reported sensitivity (ability to detect cancer when present) and specificity (ability to detect no cancer when not present) values for each modality were used in the analyses [14].
All statistical analyses were calculated using SAS® 9.4 (SAS Institute, Inc, Cary, North Carolina, USA).
The probable risk of harm to a high-risk breast implant reconstruction patient due to the presence of an imaging artifact caused by an embedded RFID-M in a silicone gel-filled breast implant was assessed. The RFID-M has a magnetic core that creates an artifact during MRI scanning, thereby preventing complete field visualization. The artifact is a cause for concern, especially during cancer surveillance procedures.
Based on the number of patients/units included in the assessments (Table 2) and the percentage of breast implants placed during reconstruction procedures of 2.5% of the Motiva Breast Implants, the estimated 4-year cumulative cancer occurrence/reoccurrence is 153 cases. In the average assumption of 20% breast implant reconstruction, the 4-year cumulative cancer occurrence/reoccurrence is 1,224 cases. RFID-M embedment creates an artifact that impedes visualization of breast implant areas ranging from 2.77% in larger sized implants to 5.37% in the most commonly used implant sizes (Table 3).
Electronic in vivo product identification has value, but the concomitant risk of failure to identify cancer due to artifact must be mitigated. It appears that adding a second imaging modality, such as ultrasound, reduces the risk of a missed cancer diagnosis because there the RFID-M does not create an artifact during ultrasonography.
The results of the artifact risk impact analyses are presented in table 4. MRI scanning will not “see” through the artifact area, calculated to be 5.37% as an average case (RFID-M in the most frequently used breast implant size). Hand-held ultrasonography can provide visualization of the artifact area. MRI provides visualization of the remaining 94.63% of the breast implant.
| Analysis Based om High-Risk Patient Cohort | Artifact Block Area = 5.37% of Implants Area (highest in Motiva Line) | Cancer Reoccurrence Rate = 2% Per Year | |||||
| Imaging Scenario | Breast Cancer Screening (Sensitivity/ Specificity) | Number of Potentially Missed Cancer Reoccurences (#/1,000) | Relative Risk of Missed Cancer Detection vs. MRI with no Artifact Risk | Number Needed to Harm (NNH); 1 patient harmed by Artifact for every "X" Screenings | Ratio: Benefit/Harm. In Vivo Traceability/ Harmed by Artifact. Based on Potential Case Counts |
| MRI - No Artifact | (0.90/0.75) | 0.3469 | 1.000 | -N/A- | -N/A- |
| MRI - Artifact Present | (0.00/1.00) | 0.5146 | 1.483 | 596 | 22.84 |
| Ultrasound -With or Without Artifact | (0.87/0.87) | -N/A- | -N/A- | -N/A- | -N/A- |
| MRI & Ultrasound - Artifact Present |
94.63% = (0.90/0.75) 5.37% = (0.87/0.87) |
0.3525 | 1.016 | 17,892 | 710.96 |
Table 4: RFID-M Artifact Impact Analysis.
Table 4 presents the results of the risk impact assessment for the high-risk patient population cohort. The results are the same for either the actual (2.5%) or the extrapolated (20%) percent of reconstruction breast implant patients. Each row represents different imaging modalities with and without RFID-M artifact when relevant. MRI and ultrasound sensitivities and specificities are in the first column. The sensitivity (ability to detect cancer when there is cancer) for MRI with artifact present is 0.00 denoting it will never detect cancer within the artifact area. Also, the specificity (ability to detect no cancer when there is none) is 1.00 at the artifact because there will never be a falsepositive. The ultrasound imagery is the same with or without RFID-M because there is no artifact during ultrasonography. The bottom row shows the percentage applied to each modality and its sensitivity and specificity. In this analysis, the MRI will only “see” the non-artifact voided area and ultrasound “sees” through the artifact voided area. This model doesn’t utilize the combined sensitivities and specificities of MRI added to ultrasound which would likely reduce false-positives more than false-negatives.
The second column shows the number of potentially missed cancer detections per 1,000 screening exams across scenarios and modalities. Note how close the rates are between MRI with no artifact and dual modality of MRI + US and artifact. Using this information, the relative risk (RR) of artifact modalities to that of MRI with artifact can be calculated. The fourth column expresses Number Needed to Harm (NNH) statistics. Using an MRI with RFID-M present and obstructing 5.37% of the breast implant image one high-risk patient with a cancer recurrence would likely be missed for every 596 high-risk patient screening exams performed. Likewise, when dual modality of MRI and US are used to study the high-risk patient group, it would take 17,892 screening exams before a patient with cancer recurrence is likely to be missed (false negative). The addition of US to the artifact void area mitigates the impact of the artifact quite substantially.
The final column shows the ratio of RFID-M 100% traceability benefit (high-risk patients with cancer not missed in imaging studies) to the harm caused by the artifact (highrisk patients with cancer missed in imaging studies). Even for the MRI study alone with RFID-M artifact, 100% traceability finds a 22.84-fold increase in the number of patients benefiting over the number of patients harmed. Dual-modality improves this ratio up to a 710.96-fold increase in the number of patients benefiting over the number of patients harmed.
FDA and the European Commission (EC) have made safety and integrity of the global healthcare supply chain a strategic priority by adopting legislation for Unique Device Identification for medical devices [15,16]. Other international regulatory bodies are expected to introduce similar product identification requirements that will harmonize with the FDA and EC laws.
Class III medical devices, such as silicone gel-filled breast implants, are required to comply with the new device identification requirements by 2024, but the requirements call for the application of product identification language and identification bar code to the device package label; direct marking is not required. Placement of a standardized UDIcompliant barcode on the device package label is also a key benefit assuming the bar code is scanned into the patient’s record and entered into the Global Unique Device Identifier Database (GUDID) [17].
The RFID-M embedded Motiva Implants® With Q Inside Safety Technology is the first and only silicone gel-filled breast implant that offers in vivo electronic product identification, a feature that goes beyond the forthcoming UDI requirement for Class III devices. Every human intervention requirement is an opportunity to break the product identification chain and possibly lead to an unnecessary explantation, as is known to have occurred due to the PIP debacle [6]. The RFID-M ESN must be entered manually into patient’s record and the patient must self-register on the company website [18]. Once registration occurs, product traceability is assured for the Motiva Implants® With Q Inside Safety Technology
RFID-M presence in a silicone gel-filled breast implant causes an imaging artifact, most notably during MRI scans. This preliminary report has shown that a possible mitigation to address the artifact is the addition of a second imaging modality. It appears that a dual modality approach, adding ultrasound after MRI, provides a diagnostic approach that increases the probability of finding a new lesion or cancer reoccurrence. A full report is forthcoming, but these early results are promising.
The unfortunate experiences of not knowing whether breast implant patients had adulterated PIP breast implants caused stress, unnecessary surgical risk and significant quality of life decrements for patients and healthcare providers [4,6]. The growing concern about ALCL and the possible linking to breast implant surface type may prove to be significant and a compelling reason why in vivo product identification RFID-M electronic identification enablement in silicone gel-filled breast implants prevents the fear of “not knowing”. A safety benefit for the patient that is irrefutable.
Concern for patient safety is real due to the RFID-M artifact, and the possibility of a missed cancer diagnosis. This paper presents preliminary information that the artifact presence does cause an imaging void of approximately 20 mm to 30 mm, that prevents complete MRI visualization of the patient’s chest wall. Also presented are preliminary results that indicate that a dual modality imaging sequence (MRI, followed by ultrasound), improves the probability of cancer detection.
Surgeons should take into consideration that their patients, depending on the region of the world, may have to pay more than average for implants with an RFID component like Q Inside Safety Technology. Patients should also be advised that, although MRI screening for silicone shell integrity is considered a best practice globally, incorporation of a second imaging modality is not. Thus, patients may have to shoulder the costs of additional imaging procedures.
The focus of this report was to quantitatively detail the overwhelming case that with a dual imaging modality, the harms due to RFID-M artifact appear magnitudes smaller than the benefits to both the individual and the population.
Further investigation is needed to completely address the use of RFID-M technology in silicone gel-filled breast implants and in other implantable medical devices, yet the preliminary results are promising. These early risk impact assessments RFID-M presences in the Motiva Implants® with Q Safety Inside Technology contributes to the overall safety of the patient when dual-modality imaging is utilized during cancer surveillance in high-risk patients.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Nelson MT, Brattain KA, Williams JM (2018) Does Electronic Identification Enablement for Silicone Gel Implants Impact Patient Safety? J Surg Open Access 4(1): dx.doi.org/10.16966/2470-0991.162
Copyright: © 2018 Nelson MT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access