Abstract
Objectives: Intraoperative nerve monitoring (IONM) with intermittent stimulation, can predict recurrent laryngeal nerve injury after the damage has been already done; on the other hand, continuous IONM (CIONM) via stimulation of the vagus nerve (VN) by the automated periodic stimulation (APS) electrode, should permit more reliable monitoring of the nerve’s functional integrity during surgery.
Methods: The advantages CIONM offers over its intermittent counterpart are indeed related to its ability to provide accurate and real-time feedback to enable the surgeon to act before damage has been inflicted on the nerve. Furthermore, these characteristics do not compromise safety and effectiveness of the surgical procedure.
Results: The indications for its use have been progressively expanding, despite the still living surrounding skepticism, and recurrent thyroid disease, thyroidectomy with neck dissection, pre-existing unilateral vocal cord paralysis, previous neck treatment and high risk for intraoperative hemorrhage, represent the main situations with strong indications for CIONM use.
Conclusions: This article provides a detailed description of this effective, patient’s safety tool. Here we enclose IONM with APS technical and practical steps, in order to give the idea of a clear awareness of its benefits and to encourage surgeons to widen their skills and knowledge about its potential use.
Keywords
Thyroid surgery; APS probe; CIONM; Recurrent laryngeal nerve injury; Technical tips
Introduction
Routine recurrent laryngeal nerve (RLN) visualization has been considered the standard of care for the prevention of nerve injury during thyroid surgery since the 1930s’ [1]; but, despite meticulous anatomical identification and preservation of the nerve, RLN injury still exists and causes voice impairment, together with the associated decrease of quality of life in terms of social, psychological and work-related problems. The incidence of nerve palsy continues to vary widely in literature, where a transient RLN palsy is reported to range between 0.4%-26%, and permanent RLN palsy can reach up to 5%-6% [2]. In this setting, RLN palsy represents a key performance indicator of thyroid surgery quality management and it depends not only on the type of thyroid disease and the type of resection but also on surgical training, skills, surgical devices used and surgeon’s personal experience [3].
Thus, over the past two decades, different medical devices have been introduced in the intraoperative surgical practice in order to improve surgeons’ performance and surgical outcomes, without worsening costeffectiveness rate. Various techniques have been described; IONM has been shown to be a good option to improve RLN identification during thyroid and parathyroid surgery, to reduce the incidence of postoperative temporary VCP and avoid bilateral VCP, with approximately 40% to 45% of endocrine surgeons using this technology in all or some cases [4]. Despite its limits, IONM use has been increasingly grown worldwide and the introduction of CIONM with APS electrode represents the promising tool able to monitor nerve functions by an almost real-time feedback by clear EMG changes representing nerve electrophysiological status [5]. Few side effects have been reported in the literature, but no absolute contraindications exist for its use [6].
Here we present our thyroid surgical technique with the use of CIONM with APS electrode. We strongly believe in its help during surgery, to improve operation outcomes and to meet patients’ needs and good postoperative functional outcomes. In this respect, our aim is to increase the knowledge about APS potential use, by explaining its limits and possible associated complications, clarifying its criteria of interpretations and indications, together with a short digression through tips and tricks of experienced surgical hands.
CIONM with APS Probe
IONM with APS technology requires surgeons’ knowledge in interpretation and correlation of laryngeal muscles action potential, together with preoperative and postoperative laryngoscopic findings, and surgical skills in APS probe positioning and intraoperative use. These pitfalls arise from the complexity of IONM with APS characteristics, and they represent the key to best accomplish this tool purpose.
Here we propose a detailed explanation of procedural steps of the IONM with APS technology, in order to best make full use of it and derive as much benefit as possible by improving thyroid surgery’s outcomes.
Procedural steps
Thyroid surgery with intraoperative nerve monitoring and continuous vagus stimulation by the APS probe in its procedural steps is a reproduction of its conventional counterpart apart from the supplementary steps of APS probe positioning/removal and RLN function control by initial and final vagus stimulation.
Procedural steps include:
- Access
- APS probe positioning on the vagus nerve
- Homolaterally to the APS positioning, division of the superior thyroid pedicle along with visualization and preservation of the external branch of the superior laryngeal nerve (EBSLN)
- Identification and preservation of the RLN and both parathyroid glands
- Completing the hemithyroidectomy and final check of RLN function by direct vagus stimulation. If total thyroidectomy is performed, then the same procedural steps will be repeated on the contralateral side starting with the removal of the APS probe and its positioning on the contralateral vagus nerve
- Closure
General setting
The position of the patients and of the surgical team: The patient is placed in a supine position on the operating table with the arms tucked at the sides. The neck should be midline and extended to reduce the working space under the pre-laryngeal muscles. The surgical team, typically composed of three surgeons, sees usually the operator standing on the patient’s diseased side as during standard thyroid surgical procedure.
Anesthesia: A proper positioning and function of the endotracheal tube are demanded to gain the best potential signal. In this setting, the anesthetist must avoid additional laryngeal mechanical manipulation mainly during intubation and extubation that could lead to discordant postoperative endoscopic findings. For instance, fixation of the vocal fold, arytenoid subluxations, and cuff-related injuries can produce vocal cord immobilization. In addition, all drugs that are known to cause neuromuscular blockage must be avoided [7].
Site and CIONM preparation: Proper connection and function of all wires and electrodes. The endotracheal tube presents ending surface electrodes and an EMG amplifier which is supposed to be correctly placed in contact with the free edges of both vocal cords, in order to close the circuit of electrical information with come from the automatic periodic stimulation of the vagus nerve. In fact, the electrical information convey from the vagus nerve, and through the RLN, reach vocal cord muscles, and their electrical characteristics are read by the EMG amplifier and screened on a monitor where they get a graphic representation. Ground electrodes and a handled monopolar stimulator probe complete the CIONM equipment.
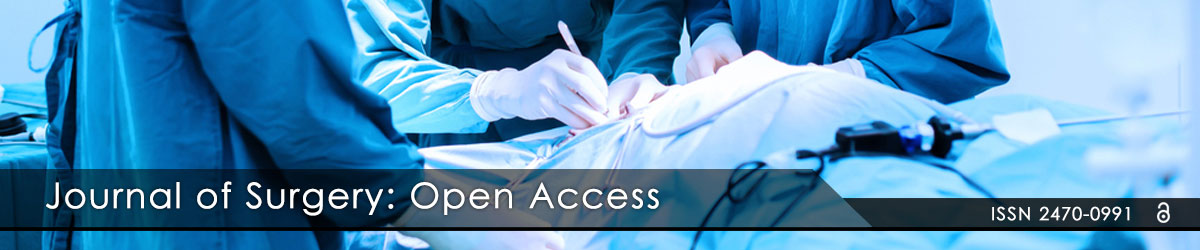
Additional surgical execution steps: After skin and subcutaneous tissues have been cut, and pre-laryngeal straps divided along the midline, before starting thyroid dissection APS electrode needs to be collocated. Its installation requires exposure of less than a 1 cm segment of the vagus nerve. The APS electrode is positioned on the nerve starting at a 45° angle and then sliding it over the vagus with the enclosure tabs open (Figures 1a and 1b). After connecting the APS electrode to the monitor, baseline values for latency and amplitude are automatically calibrated.
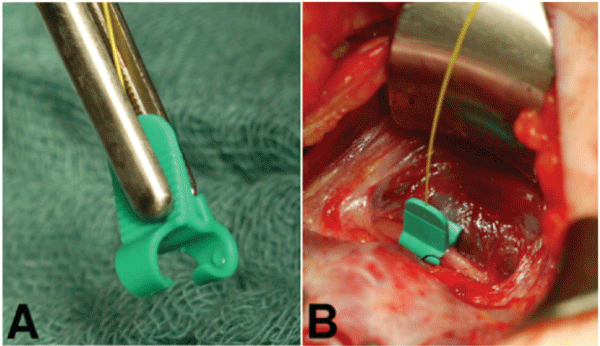
Figure 1: a) The APS electrode is first taken by a single instrument (i.e.: kelly surgical instrument) and kept open; then, b) the APS probe is positioned on the nerve starting at a 45° angle and then sliding it over the vagus with the enclosure tabs open.
Electrode installation on the vagus is technically straightforward once the surgeon’s learning curve has flattened. A baseline amplitude waveform greater than 500 IV is necessary as an initial baseline. If the amplitude and latency alarms engage early without being associated with a surgical maneuver, this is seen as artifactual and the tube needs to be subsequently repositioned and amplitude and latency baseline are recalibrated. The APS electrode is easily managed during surgery, and its dislocation is rare, whilst its dislocation could be favored by huge goiters or neck dissection because adjunctive maneuvers out of the thyroid surgical field are required.
The frequency of stimulation can be programmed, we are used to setting a low-level stimulation of the vagus nerve of 1 mA every 6 seconds (10 times/minute, pulse duration 100 µsec duration). Amplitude and latency waveforms are displayed separately and an upper limit threshold for latency and a lower limit threshold for amplitude need to be defined as separate alarm lines. In addition, acoustic signals alert if these thresholds are violated or if the electrode had been dislocated.
At the end of surgery, distal and proximal vagal stimulation is performed to exclude electrode induced vagal segmental injury, then, the APS electrode is removed.
Surgical behavior and safety: Current IONM formats allow the surgeon to intermittently stimulate and assess RLN function. While this has significant utility, such a format could potentially allow the RLN to be at risk for damage in-between stimulations [4,8]. This reality may underlie the data, suggesting that current IONM formats are limited in an ability to prevent neural injury [2,9-12]. The advantage of continuous intraoperative neuromonitoring (CIONM) is that it has the potential to monitor the entire vagus and RLN functional integrity in real-time throughout surgery and could identify electromyography (EMG) signals associated with early impending injury states [13-15]. Initial studies on CIONM with a vagal nerve electrode have suggested such monitoring is not associated with significant adverse neural, cardiac, pulmonary, or gastrointestinal vagal side effects [6,16-18]. In this setting, for continuous vagal neural monitoring to have proven utility, it must provide accurate detection of EMG changes: 1) “adverse”, associated with impending VCP, 2) “normal”, and 3) “nascent” with a resolution of adverse EMG changes with modification of the associated surgical manoeuvre. In specific, single events of decrease in amplitude more than 50% or increase in latency over 10% of the signal, represent “normal” editable and temporary EMG changes that could occur normally during surgery (Figure 2) ; on the other hand, a combination of both events, suggests the need to change immediately surgical manoeuvres to prevent a potential nerve injury, and they are classified as “nascent” if a complete normal value of both latency and amplitude is restored by a temporary interruption of any surgical stress, while, they are defined as “adverse” if a complete normalization of both parameters is not reached despite the absence any surgical activity. Furthermore, we must be able to segregate such EMG changes of impending neural injury from artifactual EMG changes that are associated with endotracheal tube malpositioning or other equipment problems (Figure 3) [19,20].
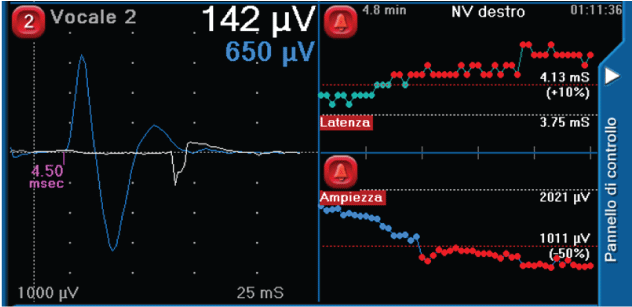
Figure 2: Example of combined “normal” editable and temporary EMG changes event of amplitude and latency changes while dissecting the right thyroid lobe.
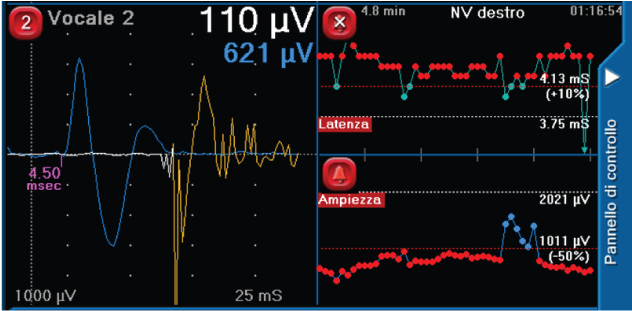
Figure 3: Examples of artifactual EMG changes due to bipolar instrument use.
In comparison to IONM, in case of RLN’s posterior branch injury, the stimulation of its external branching only, could cause falsepositive results giving an EMG signal wrongly “normal”, reflecting the performance of the inner anterior branch responsible for the vocal muscle movement [3]; thus possibly causing dyspnea at the emergence from the anesthesia. Moreover, in neurogenic lesions of the RLN due to mechanical manipulation, proximal stimulation near the larynx produces a false negative, ‘‘normal’’ IONM signal. In both cases, only vagal stimulation and, in addition, electromyographic registration of signals, which easily uncovers all kinds of artifacts, can help avoid spurious findings. Table 1 summarizes advantages and disadvantages of IONM and CIONM.
| |
IONM |
CIONM |
| Adavantages |
Faster and cheaper procedure |
Real-time nerve’s status feedback |
| |
Easy to interpret |
Gives notion about preoperative RLN electric-potential status (base-line) |
| |
|
Gives evidence of possible “type I” segmental RLN injury during surgery |
| |
|
Helps surgeons in intraoperative decision making |
| Disadvanatages |
False-positive signals due to RLN posterior
branch stimulation |
Takes longer than IONM, with higher costs |
| |
No notion about starting RLN eletric-potential status (i.e.: pre-existing imparement) |
Requires EMG knowledge |
| |
|
EMG artifacts could be linked to small hemorragia in vagus site or due to tube malposition |
| |
|
Requires adavanced anestesiologist and surgical skills |
Table 1: Advantages and disadvantages of IONM and CIONM.
Learning Curve
Performance tends to improve with experience, and in turn, it is assessed by a measure of the surgical process itself and measures of outcomes. The former include operative time, blood loss and technical adequacy (considering APS probe positioning, together with its dislocation or malpositioning, and adequate baseline values). The latter include duration of hospital stay, morbidity rates considering vagal and RLN injury, carotid artery or internal jugular vein damage, and ability to interpret and correlate EMG signals with clinical signs. The learning curve of the surgical procedure goes through the following phases: the starting point with the new step of APS probe positioning, curve ascent consisting in the improvement of performance, then the adequate level of proficiency and safety allowing the independent procedure.
There is no a threshold of the minimum number of operations to perform in order to gain a good manually, patients’ functional outcomes and good knowledge of EMG signs interpretations represent the keys of the well-trained surgeon.
One of the most important advantages of CIONM is further advancing of the procedure and expanding its selection criteria, overcoming the limitations of pre-existing vocal cord palsy, of previously irradiated or treated neck fields, of cases needing neck dissection and/or in the case of hyperthyroidisms. Strong and mild recommendations for CIONM use are listed in table 2.
| Recommendations |
Strong |
Mild |
| |
Pre-existing vocal fold palsy |
When hemithyroidectomy is needed |
| |
Previously treated neck field (surgically treated or irradiated) |
Multinodular goiter |
| |
In case of high risk for intraoperative RLN injury (i.e.: presence of diseased lymph node at level VI) |
|
| |
In neurologic patients |
|
| |
Voice professionists (i.e.: singers, teachers, loyers, etc..) |
|
| |
Hyperthyroidisms |
|
| |
Locally advanced thyroid cancer |
|
Table 2: Strong and mild recommendations for CIONM use during thyroid surgery.
Discussion
CIONM has demonstrated to be a tool capable of minimizing the risk of RLN injury; in fact, CIONM could herald, through surveillance of EMG changes, forthcoming nerve injury to surgeons who can modify the indicated surgical maneuver in order to avoid permanent nerve damage [8]. Although several studies in literature propose CIONM as a promising tool in reducing thyroid surgery’s neurologic complications, there is still the need to identify the latency and/or amplitude changes thresholds indicative of imminent RLN injury. Moreover, several pitfalls [3] continue to limit the utility of this novel technology, such as the absence of a consensus about which types of electrodes should be used for EMG registration (intralaryngeal vocal fold electrodes, trans cricothyroid membrane needle electrodes, tube surface or post cricoid surface electrodes), which is the best method for recording nerve action (continuous registration of spontaneous nerve action versus discontinuous electrical nerve stimulation), and which quantitative EMG parameters should be selected as predictive of postoperative vocal cord dysfunction (supramaximal stimulation versus minimal stimulation for RLN stimulation at the end of the operation). In fact, even if the accuracy of this tool is almost 99% when a cutoff of 200μV is used, in the case of abnormal EMG signals, the ability of IONM to predict postoperative VFP is less robust, with a PPV of 72.4% [20]. However, if an abnormal signal is attained after dissection of the RLN, the chance of some degree of VFP is higher than in the case of same values of baseline. In this situation, the surgeon can reevaluate the extent of surgery on the second side and consider electing for a second-stage procedure, depending on the nature of the operation.
Despite these still existing limits and pitfalls, practice is helping surgeons to improve this tool use by increasing patients’ benefits. In addition, in the perspective of drawing future guidelines of surgical thyroid techniques and surgical steps, preoperative and postoperative examinations should be considered belonging to the patient management [21]. Accordingly, The American Head and Neck Society itself have published guidelines for the management of invasive thyroid cancer which state that IONM provides important both intraoperative and postoperative information and for this reason they recommend its use in all cases of thyroid cancer [21]. Moreover, the American Thyroid Association Surgical Affairs Committee consensus statements have recently published that neural monitoring is confirming intact neural function at the time of surgery and it does impact on discharge planning [10]. Thus, it is widespread that the true incidence of temporary and permanent postoperative VFP may be underestimated if a routine laryngeal examination is not performed.
Conclusions
In this setting, a protocol to correlate clinical aspects with intraoperative findings must be developed and followed. In conclusion, to better combine surgical technique and patients’ outcomes, further studies including highquality multicenter, prospective, randomized trials are required to verify the advantages of interest.
Conflict of Interest
Giuditta Mannelli and other co-authors have no conflict of interest.