
Figure 1: This picture shows the “Gummy bear” consistency of gel fill in the modern gel implant. Note how despite the rent in the shell, the fill retains its shape and does not flow out.
Umar Choudry*
Division of Plastic Surgery, University of Minnesota, Minneapolis, Minnesota, USA*Corresponding author: Umar Choudry, University of Minnesota, 420 Delaware Street, MMC 195, Minneapolis, MN 55455, USA, E-mail: choud008@umn.edu
Breast Implants are one of the most commonly utilized medical prostheses in surgical procedures performed today. The main use is in breast augmentation (enhancing the female breast) and breast reconstruction (recreation of the female breast). Many surgeons and physicians deal with a variety of breast problems ranging from pathology to cosmesis. Having a good understanding of the up-to-date information regarding breast implants is important to deal with questions or concerns from patients when they arise. This review article will highlight some important aspects concerning breast implants; and give a general overview about the manufacturing process and device characteristics, the current issues surrounding implants, and the scope of their use in breast surgery.
Breast implants; Manufacturing; FDA; Controversies; Indications
Breast implants have been instrumental in changing the face of breast surgery over the last few decades. One of the most widely utilized medical devices in plastic surgery; they are used to either augment the size of a natural breast, or used to recreate the breast mound in breast reconstructive cases. Through the years, few medical devices have received more scrutiny from the FDA (Food and Drug Administration) in particular, but also by politicians, the media, and the public in general. To this day, the erroneous perception of the association of breast implants with numerous systemic diseases still lingers, despite ample scientific data refuting this. Like any man-made device, breast implants do have imperfections: they leak, rupture, get infected, and possibly need replacement. However, that is true for any other medical device as well. Importantly, technological advancements in breast implant device construction have led to improved device safety profiles and a decrease in device-related complications. This review article will provide an overview of breast implant technology, some popular controversies associated with breast implants, and the basic indications for their use in plastic and reconstructive surgery.
Enhancing the female breast is not a modern idea. Czerny was the first person to attempt this back in 1895, when he transferred a lipoma from a patient’s back to her breast to correct asymmetry [1]. Prior to the era of the modern breast implant, numerous materials have been used to “fill” the breast. Fat, paraffin, glass, ivory, sponges, and rubber have all been used at one time or another [2]. Needless to say, many were associated with devastating complications. Dr. Thomas Cronin, et al. [3] are credited with developing the first breast prosthesis in 1962. As the story goes, at the time, blood banks in his hospital had recently switched from storing blood in glass bottles to plastic bags. One of Cronin’s colleagues noted that the feel of a plastic bag of blood was akin to the softness of a female breast. It was not long thereafter Cronin was introduced to a product, Silicone, at a medical meeting. He learned that it was a versatile product that was well suited for medical manufacturing. He immediately connected the ‘off the cuff remark’ of his colleague to the development of a ‘breast implant’. The first prototype was implanted in a dog named Esmeralda, and then shortly thereafter, successfully in the first human subject, Timmie Lindsey.
Breast implants are basically a silicone shell with either saline or gel filling. Silicone has properties that are favourable for its use in breast implants. It is inert, stable, capable of sterilization, and is easily available. More importantly, it can be produced in a number of forms, including, gels and solids, depending on the degree of cross-linking. Thus the outer shell of the gel breast implant is a vulcanized silicone elastomer, and the ‘gel’ filling is polydimethylsiloxane (PDMS), a polymer of silicone. On the other hand, saline implants have salt-water as their fill material. It is, therefore, worth noting that the term ‘saline implant’ does not imply the absence of silicone in the device, as in fact the shell is made of silicone. In the United States, currently only silicone and saline filled implants are available for use in patients. Other products like oils have been used as implant fills but have not received FDA approval for general use in the United States.
Over the years, this device has evolved tremendously. Manufacturers, biomedical engineers, and surgeon scientists have learned the weaknesses of each generation of implant. Clinical outcome data has guided the manufacturing processes in an effort to produce the ‘ideal’ breast implant, which has still eluded us. These changes and advances in design have been based on characteristics of its shell, fill, shape, and surface properties.
Over the years of evolution of the silicone breast implant, the characteristics of its shell and gel-fill have been re-formulated in an effort to produce less gel-associated problems and improved longevity of the product. The ‘first’ generation product (1960s) had a thick shell, and thick viscous gel. The main issue with this implant was the associated high rate of capsular contracture (a phenomenon in which the scar tissue around the implant becomes thicker and tighter, resulting in a painful and distorted breast). The ‘second’ generation implant (1970s) was designed to decrease the rate of capsular contracture by using a thinner shell and less viscous gel. Despite this change, the contracture rates remained high, and in fact the rupture rates increased due to the weaker shell construct. Another issue that became apparent was that of ‘gel-bleed’, the ‘leakage’ of silicone oil (non-crosslinked silicone) across the shell into the breast tissue. The ‘third’ generation (1980s) implants aimed to target those shortcomings by introducing thicker, multilayered, chemically reinforced barrier shells, with cohesive gels as the filler. Subsequent generations of implants (‘fourth’ – 1990s, and ‘fifth’ – current) have continued to refine the basic structural principle of the third generation implants, with the latest devices having a form-stable gel-fill (gummy bear consistency) (Figure 1). The current implant generations are less prone to capsular contracture and leaks with lower rates compared to their earlier generation counterparts. Implant rupture rates are reported to be between 6-15% at 8-10 years; and capsular contracture rates are reported to be between 8-25% at 8-10 years (rates vary based on different implant products, as well as different clinical scenarios in which the implants are used e.g. primary vs. secondary procedures, augmentation vs. reconstruction procedures) [4].

Figure 1: This picture shows the “Gummy bear” consistency of gel fill in the modern gel implant. Note how despite the rent in the shell, the fill retains its shape and does not flow out.
Breast implant devices may either be smooth walled or ‘textured’ (a rough surface so as to prevent movement of the device, better tissue ingrowth, and possibly reduced capsular contracture rate) (Figures 2 and 3). There are two main techniques to accomplish texturing: negative contact imprinting and coating with salt crystals. Negative contact imprinting results in multiple small nodule formations on the surface of the device. The second method employs coating the device with salt crystals under pressure. Through the process of development of the implant, these crystals detach leaving small depressions on the surface. Apart from certain specific indications (eg, anatomic implants (discussed below), and tissue expanders) where textured implants are the only choice, surgeons usually choose the implant texture based on their own personal experience with the device.

Figure 2: This picture shows a smooth, round gel breast implant.

Figure 3: This picture shows a textured surface, round gel breast implant.
Breast implants can either be ‘round’ or ‘shaped’ (also known as anatomic) (Figure 4). Round implants, as the name implies, have a circular circumference. In an effort to get a more anatomic breast shape during augmentation or reconstruction, shaped implants were developed. These devices are teardrop shaped, with a greater projection in the lower half of the device. To prevent rotation of the device in the breast pocket, the surface is always textured. Again, it is surgeon experience and preference that usually dictates what kind of implant he/she advises. No conclusive data show the superiority of one over the other in terms of outcome.

Figure 4: This picture shows a textured surface, anatomic (shaped) gel breast implant.
The association between implants and systemic diseases, like connective tissue disorders and autoimmune diseases, has continued to be debated in the media and a section of the general public for many years despite overwhelming scientific data to the contrary. The worry was that the early generation implants, (which had thinner outer shells that were prone to rupture, and also had viscous gel filling that could flow), when ruptured, could result in leakage of gel into the breast tissue. This leaked gel could then absorb into the body and result in immune reactions or direct injury to the tissues. There have been no data to date that suggest this potential leakage causes any major systemic illness. Multiple largescale epidemiological studies have consistently shown no evidence of the association of breast implants with autoimmune diseases, Lupus, Scleroderma, or other rheumatologic disorders [5-8]. The leakage of silicone, however, could result in local foreign body reactions within the breast tissue or regional lymph nodes. This may result in problems like granulomas, distortion of the breast, palpable masses, and even pain, which necessitates excision of the leaked gel and replacement/removal of the failed implant. No scientific data have shown that these local problems are associated with any systemic illnesses.
Multiple long-term, large-scale epidemiological studies have consistently showed no carcinogenic effect of silicone on the breast or any other tissue [7-15]. The other main concern pertains to the possibility of implants masking breast cancer on mammograms due to their radiopaque nature. Again, scientific data do not support this concern for delayed detection of malignancy. The ASPS (American Society of Plastic Surgeons) and ACS (American Cancer Society) recommend standard mammography schedules for patients with breast implants, but do recommend additional displacement views (Eklund views) in addition to the standard 2-view mammogram [16,17].
Recently, the association between breast implants and ALCL (Anaplastic Large Cell Lymphoma) has come to light. In fact, in January 2011, the FDA issued an alert on the matter [18]. There have been a few case reports and small case series describing this association in the last few years [19-35], but no large scale epidemiologic studies showing a strong correlation or any evidence suggesting causality have been published. At this time, based on current scientific knowledge, experts in the field state there may be a very weak correlation between development of ALCL and breast implants, somewhere between 0.1-0.3 per 100,000 women with breast implants per year, but are not willing to state that there is a causal association [27,36]. Further research is being conducted to better understand this potential issue.
When breast implants first came to the market in the 1960s, the FDA (Food and Drug Administration) was not charged with regulating medical devices. In 1976, the medical device regulation act was signed into law, thereby charging the FDA to provide oversight on safety and effectiveness of medical devices. When reports of women developing systemic complaints of rheumatologic nature began surfacing in the 1980s, the FDA placed a moratorium on the use of silicone breast implants in 1992 due to lack of scientific evidence supporting the safety and efficacy of silicone breast implants. The major manufacturers of the devices were encouraged to begin long-term data collection under strict FDA approved studies. Through most of the 1990s and early 2000s multi-institutional trials were performed and periodically reviewed. Ultimately in 2006, the FDA approved the use of silicone breast implants for general use in the U.S. market. It is worth noting, that during the almost 15 years of moratorium on silicone implants in the U.S., the rest of the world continued to use them almost exclusively, and even in the U.S. the moratorium affected only cosmetic primary breast augmentation. In other words, breast reconstruction, lifts, and congenital breast cases could still utilize silicone implants.
Enhancing, rejuvenating, and/or recreating the female breast is a challenge for the plastic surgeon. Breast implants play a vital role in this endeavor by providing the structural fill necessary to augment or reconstruct the breast mound. The aesthetics of the breast are complex given that there is no clear definition of what makes the perfect breast. These definitions vary from person-person, region-region, and even through the ages. Plastic surgeons are involved in breast surgery when the anatomy of the breast is deranged whether from the effects of aging, lactation, trauma, infection, or cancer therapy. Their aim is to recreate as ‘normal’ as possible the form and aesthetics of the breast under the individual circumstances the patient presents with. Thorough knowledge of anatomy, wound healing, tissue handling and manipulation, as well as implant technology are vital for a successful outcome.
Breast enhancement surgery is the most popular cosmetic surgical procedure performed today [37]. Almost 300,000 procedures were performed in 2012 in the United States. The most common method to accomplish the augmentation is the utilization of breast implants (other methods include fat grafting and suction devices). The implants are placed behind the natural breast tissue through incisions made in the breast fold, around the areola, or even in the axilla (Figures 5 and 6). Where indicated, these cosmetic procedures may also require the breast tissue to be ‘lifted’ (mastopexy) simultaneously to centralize the nipple-areolar complex over the implant (eg, in droopy, deflated breasts). There are numerous other parameters that the surgeon has to contend with prior to the placement, including the type of implant (fill, surface, shape), location of placement (sub-muscular, sub-glandular), and size of implant, which will impact the final outcome. These are decided based on patient preferences, anatomy, and surgeon experience.
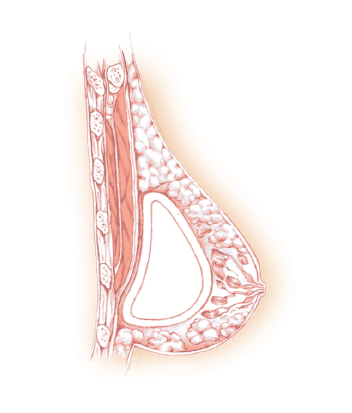
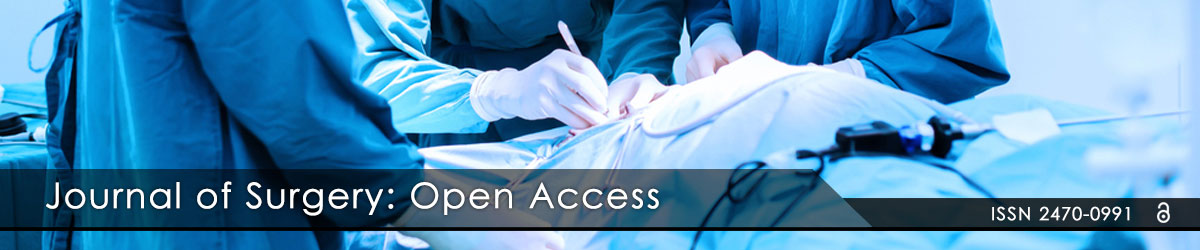
Figure 5: This drawing represents the sub-glandular (under the breast tissue) placement of a breast implant.
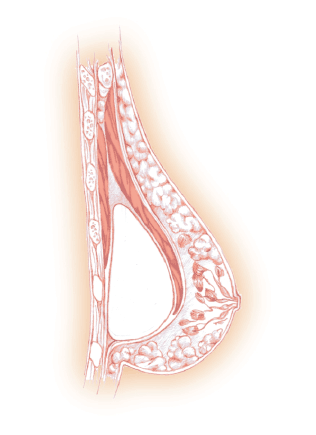
Figure 6: This drawing represents the sub-muscular (under the pectoralis major muscle) placement of a breast implant.
Breast cancer is the most common malignancy (excluding skin cancers) in women with over 90,000 breast reconstructions performed in 2012 in the United States [37,38]. The use of breast implants is the primary form of breast reconstruction in the United States, with 78.5% of reconstructions performed utilizing these devices [37]. The remaining 21.5% are performed by transferring the patient’s own tissue from either the abdomen or back (flaps), and sculpting it into a breast mound. There are a number of ways in which breast implant devices are used in breast reconstruction. If the patient has had a ‘skin-sparing mastectomy’ (removal of only the breast tissue without removal of all/most of the skin tissue) then a direct implant could be placed to reform the breast mound. However, if the patient has had a traditional mastectomy with most of the skin removed with the specimen, or is being evaluated for a ‘delayed’ reconstruction (the mastectomy was done in the past and the patient has a flat, well healed chest without a breast mound), then one first has to ‘stretch out’ the skin on the chest to make room for the breast implant. Therefore, one initially has to place a tissue ‘expander’ (an inflatable, balloon-like device) in the chest wall (Figure 7). This expander is serially filled with saline in the clinic setting over a period of weeks, until the desired breast size is formed. Thereafter, at a second procedure, the expander is removed and the permanent breast implant is placed, thus recreating the breast.
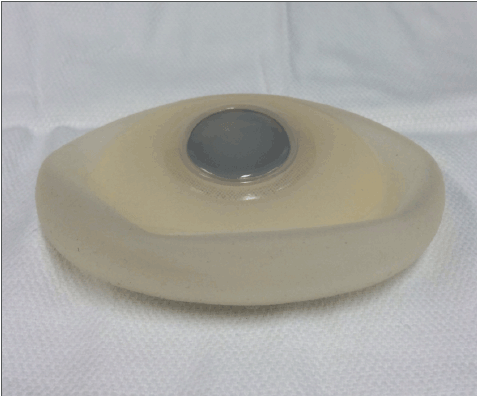
Figure 7: This picture shows a textured surface, breast expander. The circular portion in the center of the device is the integrated port where the surgeon percutaneously injects saline into the implant.
Over the years, just as there have been advances made in breast implants as described previously, tissue expanders have had their share of improvements. Integrated ports, self-sealing technology in case of accidental needle punctures, self-filling expanders, remote controlled gas filling expanders, and all-in-one expander/implant devices have been developed and/or are being studied. In the last few years, attention has once again turned to fat grafting as a useful adjunct to enhance the overall aesthetics of the reconstructed breast. Injection of highly refined autologous (the patient’s own) fat in small aliquots into the soft tissues surrounding the implant ‘softens’ the look of the breast, hides the outline of the implant in thin patients, and “fills in” contour abnormalities created by scar contracture.
Currently breast implants are vital devices for breast enhancement and reconstruction. The market for these devices is strong and expanding, especially in the United States. However, advances being made in the fields of tissue engineering, stem cell research, and fat grafting may potentially have a huge impact on the future of breast surgery and implants. Despite the great technological advancements in the manufacturing of these implants, the ‘perfect’ implant still eludes us. Implant rupture, capsular contracture, and reoperations for a multitude of implant related issues (malposition, size change) continue to occur. Additionally, the FDA was historically very concerned about the safety and efficacy of breast implants, and continues to monitor these devices closely. Through scientific studies many of the anecdotal associations with systemic diseases have been debunked. However, the recently described potential association with ALCL has again caused doubt in the authorities regarding the safety of these implants, despite this occurrence being extremely rare. As a general rule, we as scientists and physicians must continue to strive for medical progress and advancement through research and development; but we must also keep an open, critical, and skeptical mind to ensure the safety of our patients and improve outcomes based on scientific data.
Download Provisional PDF Here
Article Type: Review Article
Citation: Choudry U (2016) Breast Implants–Current Insights on a Common Medical Device. J Surg Open Access 2(4): doi http://dx.doi.org/10.16966/2470-0991.128
Copyright: © 2016 Choudry U. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access