
Figure 1: PEMF Generating Device.
MSCs are exposed to a 15 Hz, 2.4 mT uniform PEMF for 20 min/day, 3x/week for 21 days. Field generated by a Helmholtz coil. Frequency detected by oscilloscope and field strength measure by Gauss meter.


Christina L Ross*
Department of Regenerative Medicine, The Wake Forest Institute for Regenerative Medicine, The Wake Forest Center for Integrative Medicine, Medical Center Blvd. Winston-Salem, North Carolina, USA*Corresponding author: Christina L Ross, The Wake Forest School of Medicine, Institute for Regenerative Medicine, 391 Technology Way, Winston-Salem, Carolina, USA, Tel: +1-336-713- 7274; Fax: +1-336-713-7290; E-mail: chrross@wakehealth.edu
Osteoporosis affects half the population of people over 60 in developed countries. Various anabolic, anti-resorptive, hormone replacement therapies and bone grafting have been used to retain healthy bone mass and strength; however, they can produce serious adverse effects. To test alternative therapies to drugs, we evaluated the effect of FDA approved 3-D biodegradable poly-L-lactide acid scaffolds seeded with both osteoblasts and mesenchymal stromal/progenitor cells exposed to a 15 Hz, 2.4 milli Tesla pulsed electromagnetic field to promote osteogenic bone regeneration by stimulating osteogenesis. Once adhered, cell scaffolds were evaluated for active osteogenic bone proteins to provide evidence that PEMF accelerates osteoblast and MSC proliferation, differentiation, and mineralization. Evaluation of active osteogenic markers showed pulsed electromagnetic field increased levels of osteocalcin, osteopontin, and alkaline phosphatase in cell therapies up to 30%. An increase in the rate of osteogenesis was observed between 7-14 days, providing a window of efficacy for combining both pulsed electromagnetic field therapy with cell-seeded tissue engineered scaffolds. The combination of PEMF with bioengineered cell therapies can provide alternative treatments to current problems of limited tissue availability, donor site morbidity, immune rejection, and pathogen transfer due to autograft.
Pulsed Electromagnetic Field; Mesenchymal Stromal Cells; Bone Tissue Engineering; Osteoporosis; Osteogenesis
PEMF: Pulsed Electromagnetic Field; MSCs: Mesenchymal Stromal Cells; BTE: Bone Tissue Engineering; HRT: Hormone Replacement Therapy; PTH: Parathyroid Hormone
Osteoporosis is a skeletal disorder characterized by compromised bone strength, increasing the risk of fracture. Besides impaired quality of life, patients experience increased mortality rates in conjunction with osteoporosis [1]. Current estimates predict that in developed countries, half of the population over the age of 60 will suffer from osteoporosis [2], with 17 billion dollars being directly spent on the care of osteoporotic fractures in the U.S. in 2005, predicting osteoporosis-related direct costs would increase to 25 billion dollars by 2025 [3]. Pharmacological therapies such as hormone replacement therapy [HRT], and anabolic and antiresorptive drugs are currently being used to treat osteoporosis, but adverse effects of different treatments such as hormone replacement therapy [HRT] may induce high incidence of breast cancer, depending on the length of therapy and age of the patient [4]. Biophosphates for the treatment of osteoporosis have been reported to cause osteonecrosis of jaw bone structure [5], while long term use of parathyroid hormone [PTH] has received the US Food and Drug Administration’s “black box” warning regarding osteogenic sarcoma [6]. Bone allograft and autograft have also been used; however, pathogen transfer due to autograft [7], and immune rejection due to allograft [8] cause complications for these procedures.
Alternatives to treating osteoporosis without using drugs or bone transplantation include adult stem cells, which have been used in the clinic for over a decade, mainly to treat tissue injury and immune disorders. In particular, mesenchymal stromal/progenitor cells [MSCs - sometimes referred to as mesenchymal stem cells] derived from adult bone marrow, provide a promising stem cell poplulaton for bone repair in skeletal disease due to their immuno-suppressive and immune-evasive properties [9]; however, their consistency in regenerating bone tissue is still under investigation. A growing body of data indicates that stem cell function is critically influenced by exogenous signals from the extracellular microenvironment [10,11].
Recent advances in pulsed electromagnetic field [PEMF] therapy to effect osteoporosis report a decrease in the deterioration of trabecular and cortical bone microarchitecture [12], as well as improved bone mechanical properties such as maximum load, stiffness, and elastic modulus [13]. This effect involves a series of responses from osteoblasts and their progenitor cells. Another non-pharmacological therapy for the treatment of osteoporosis is bone tissue engineering [BTE], which involves inducing newly functional bone regeneration through the synergistic combination of biomaterials, cells and growth factors [7]. In this study we used MSCs seeded onto a BTE scaffold, and exposed to PEMF to determine how these three combined therapies could provide a more effective means of stimulating bone regeneration. MSCs were used because they easily adhere to polymers in tissue culture [14], and their high proliferative rates, combined with their ability to withstand freezing temperatures, allows for their expansion in clinically relevant numbers [15]. They also have a tendency to home to damaged tissue sites that are expressing inflammatory molecules, suggesting a higher efficacy of engraftment into inflamed tissues. Cultured MSCs can be stimulated using differentiation media [DM] to influence tissue type [16]. The application of an appropriate physical stimulus such as PEMF to MSCs in culture has been reported to overcome challenges associated with standard culture systems, namely limited diffusion, non-homogeneous cell-matrix distribution, and reduced cell proliferation and differentiation [17]. The addition of 3-D biodegradable polymer scaffolds to enhance the formation of bone has been reported to support the accumulation of, and increase the dose of MSCs directly to injured tissue sites [18]. While the contribution to osteogenic differentiation has already been established for poly-L-lactic acid [PLLA] based BTEs [19] and MSCs [20], the aim of this study is to investigate whether PEMF can expedite the osteogenic process using these BTE and MSC methods to form bone.
Osteoblasts: As control, ATCC [Manassas, VA] fetal osteoblasts [FOB] were cultured in a mixture of Ham’s F12 Medium Dulbecco’s Modified Eagle’s Medium and 2.5 mM L-glutamine [without phenol red]. To make the complete growth medium, 0.3 mg/ml G418 and 10% fetal bovine serum [FBS] were added to base medium. Cells were incubated at 34° C, with 5% CO2 , per manufacturer’s instructions. Cell populations were grown to 80% confluency. To simulate the in situ environment, growth factors used to enhance osteogenic differentiation included: 20 mg/ml transforming growth factor-beta [TGF-β]; 20 mg/ml vascular endothelial growth factor [VEGF]; and 20 mg/ml bone morphogenic protein [BMP-2].
Human BM-MSCs: Mesenchymal Stem Cell Growing Media and supplements [MSC-GM, Lonza, Walkersville, MD] were used for culturing previously characterized hBM-MSCs [21]. Cells were cultured in T-75 flasks using 36 ml of media per flask, incubating at 37° C, with 5% CO2, and grown to 100% confluency before osteogenesis was induced. To stimulate osteogenesis, differentiation [induction] medium [DM] was used combining DMEM low-glucose [Invitrogen, Carlsbad, CA], 10% FBS, 100 nM Dexamethasone [Sigma, St. Louis, MO] 10 nM β-glycerophosphate [Sigma], and 0.05 mm 2-phospho-L ascorbic acid [Sigma]. Media was changed every 3-4 days for 3 weeks by completely replacing the medium with fresh osteogenic induction media.
BTE Scaffold and Cell seeding: Poly-D, L-lactide acid [PLLA] Bio Mesh [Biomedical Structures, Pinebluff, NC], having 83.5% porosity and 12.1 µ mean size, was used as the scaffold base. This particular polymer was chosen for both its strength and flexibility, similar to natural bone [22]. To avoid degradation, the biomesh was kept in -80° C until ready for use. Mesh was cut using sterile forceps and scissors to fit 1cm2 squares into a silicone sandwich frame. Sterilization was performed with ethylene oxide [EtO]. Using a 5 ml pipette, scaffold was soaked in phosphate-buffered saline [PBS] and 20 mg/ml fibronectin [Thermo Fisher Sci, Waltham, MA], until well absorbed. The scaffold material is somewhat hydrophobic, so soft pressure is needed to soak it thoroughly without damaging the fibers. After saturation, scaffold was incubated a minimum of 1 hour, then PBS was aspirated off. Cell media was then pipetted into a non-tissue treated 150 mm plate, and cells were seeded onto scaffold at 10 × 106 cells/ cm2 . Media was added up to center of silicone sandwich mold, making sure scaffold and frame were not floating. Cell scaffolds were divided into 4 groups: 1] Osteoblasts with differentiation media [DM] only [FOB + DM]; 2] Osteoblasts with DM exposed to PEMF [FOB + DM + PEMF]; 3] MSCs with DM only [MSC + DM]; and 4] MSCs with DM exposed to PEMF [MSCs + DM + PEMF].
PEMF Treatment: Cell-seeded scaffolds, in frames, were taken directly from incubators in plates including media, and were placed in 34° C [osteoblasts] or 37° C [MSCs] water bath, and exposed to a 15 Hz, 2.4 mT uniform PEMF generated by a Helmholtz coil (Figure 1), for 20 min, 3x/ week for 3 weeks. This time point was chosen because of its previously reported successful effects on the stimulation of osteogenic differentiation [23]. Using a previously characterized BM-MSC line [21], our aim was to show that PEMF can expedite the differentiation capacity of MSCs seeded on a FDA approved biodegradable PLLA scaffold for structure. Cell DM contain glycerophosphate, ascorbic acid, and dexamethasone, which have been used to preserve cell viability up to 90% in cells kept outside CO2 incubators for up to 24 h [24]. Both osteoblasts and MSCs had controls from same cell lots kept in incubator. All PEMF treatments were given immediately after media change. Supernatant samples were taken at days 0 [24 h], 7, 14, and 21.

Figure 1: PEMF Generating Device.
MSCs are exposed to a 15 Hz, 2.4 mT uniform PEMF for 20 min/day, 3x/week for 21 days. Field generated by a Helmholtz coil. Frequency detected by oscilloscope and field strength measure by Gauss meter.
Morphological Assessment: Morphological assessment to detect cell attachment to scaffold included images taken using confocal microscopy [Olympus Fluo view FV 10i] to assess cell attachment by staining cell nucleus with Dapi [concentration of 1:1000 ratio dapi to PBS]. Cell viability was determined using live [green]/dead [red] calcein kit [Molecular Probes, Eugene, OR], per manufacturer’s instructions. All images were taken with a 10x objective. Scanning electron microscopy [S.E.M.] was used to detect bone formation and tissue matrix. All images were taken at day 0 [24 h], 7 days, 14 days, and 21 days.
Bone Protein Assessment: Enzyme-Linked Immunosorbent Assay [ELISA] kits: Osteocalcin kit [Invitrogen, Carlsbad, CA]; Osteopontin kit [Ray Bio Norcross, GA], and Alkaline Phosphatase kit [Ray Bio] were performed per manufacturer’s instructions using cell supernatant samples that were frozen in -80° C until ready for use. Once tested, samples were read on a micro titer plate at 450 nm.
Statistical Analysis: Experiments were performed for a total of four trials. The results are expressed as mean ± standard error of mean. Oneway ANOVA was used to determine statistical differences between the ELISA groups, with p < 0.05 considered significant.
Bone remodeling is a highly integrated process of resorption by osteoclasts and formation of bone tissue by osteoblasts, which results in precisely balanced skeletal mass with renewal of the mineralized matrix. Biomineralization is the process by which hydroxyapatite is deposited in the extracellular matrix [ECM] [25]. Ideal parameters for regenerating bone would include biocompatible scaffolds that closely mimic the natural bone ECM, MSCs, or osteogenic cells necessary to lay down the bone tissue matrix. Morphogenic signals also help to direct the cells to the phenotypically desired type [7].
In this study, cells exposed to PEMF treatment over a 21 day period showed improved aggregation and attachment compared with controls. Both FOB vs FOB + PEMF and MSC vs MSC + PEMF show optimal attachment at between 7-14 days (Table 1). Live/dead assessment over a 21-day period appears to be most viable at 7-14 days as well (Table 2). Scanning electron microscope [S.E.M.] used to assess bone formation and tissue matrix after exposure to PEMF, show more effective differentiation of both FOB and MSC to bone-like structure between 7-14 days when exposed to PEMF for 20 min/day 3d/wk for 21 days, compared with controls (Table 3). After 21 days cells became osteoclasts [bone resorption], which is defined as the process by which osteoclasts break down bone and release the minerals, resulting in a transfer of calcium from bone fluid to the blood.
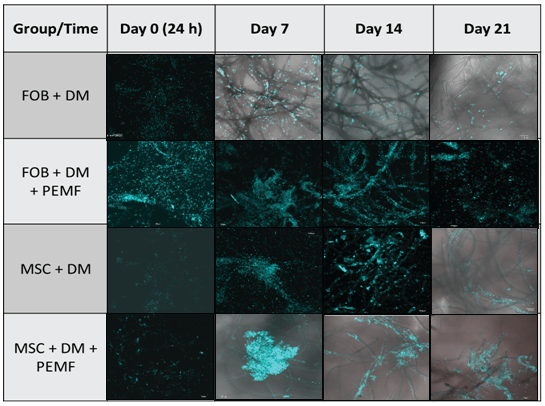
Table 1: Cell attachment: Cell attachment to PLLA scaffold was assessed using DAPI to stain cell nuclei. Images were taken at 10x using confocal microscopy Olympus Fluoview FV 10i imaging system using scale bar set at 100 µm. Optimal cell aggregation and attachment appears between 7-14 days after cells seeding, when exposed to PEMF.
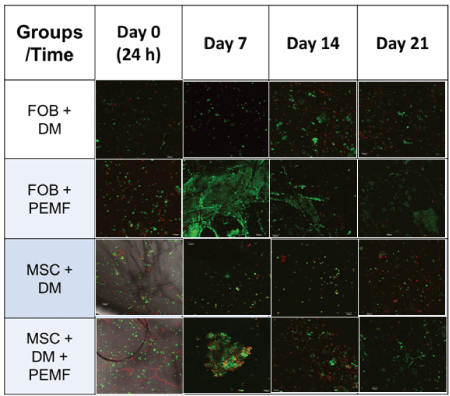
Table 2: Cell Viability: Cell viability was assessed using live/dead staining technique (live-green, dead-red) and imaged at 10x using confocal microscopy Olympus Fluoview FV 10i imaging system with scale bar set at 100 µm. Cells appear most viable between 7-14 days after seeding onto PLLA scaffold and exposed to PEMF.
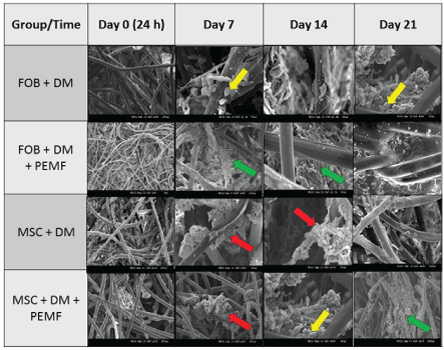
Table 3: Cell Osteogenesis: S.E.M. images (100 - 200 µm) show greater osteogenetic formation of bony-like structure occurring between 7-14 days after seeding cells onto PLLA scaffold. Green arrow shows collagen fibers, red arrow shows calcification and yellow arrow shows vesicle matrix.
Active markers of osteogenesis were assessed and compared with bone marrow derived osteoblasts to determine bone formation processes. These markers include synthesis of bone matrix and formation of dense mineralization. Both osteoblast and MSC scaffold samples were tested for Osteocalcin [OCN - a marker of formed bone tissue], Osteopontin [OPN - anchors bone cells to the mineralized bone surface], and alkaline phosphatase [ALP - makes phosphate available for calcification]. All appear to be increased through exposure to the PEMF in the 7-14 day time period (Figure 2). Highest increases in protein levels of OCN (Figure 2a), OPN (Figure 2b), and ALP (Figure 2c) were 3-fold in the osteoblasts, and in 1.5 fold in MSCs after exposure to PEMF treatment were shown between days 7 and 14, during the 21 day study. Data (Figure 2d) shows mean ± SD for different bone proteins and time points.
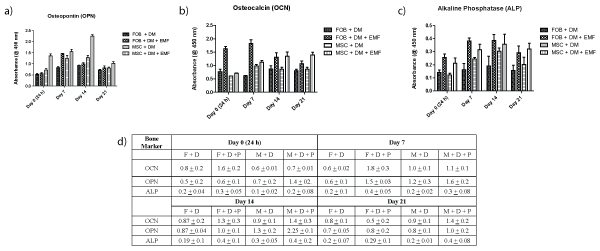
Figure 2: Production of OCN, ALP and OPN bone proteins. Both osteoblast and MSC scaffold samples were tested for
a) OCN.
b) OPN.
c) ALP
All protein levels detected using ELISA. Increased levels of these three bone markers are greatest between days 7 and 14.
d) OCN, OPN, and ALP levels using ELISA.
Increased levels of these three bone markers are greatest between days 7 and 14.
Osteogenesis is a complex series of events by which bone marrow [BM] derived MSCs differentiate to generate new bone. Temporal and functional patterns of bone protein expression characterize the osteoblast maturation process, which can be divided into proliferation, differentiation, and mineralization stages. OCN, or bone Gla protein [B.G.P.], is the major non-collagenous protein of the bone matrix. It is synthesized in the bone by the osteoblasts, whereby OCN levels reflect the rate of bone formation [26]. OPN has been shown to improve bone toughness, suggesting its importance in preventing crack propagation, thereby affecting bone mass, structure, and matrix porosity [27]. As an active marker of osteoblasts and hard tissue formation, alkaline phosphatase [ALP] is crucial to the mineralization process [28]. In osteogenesis, success is measured by robust expansion of ALP, leading to mineralization of the neotissue [29,30].
BM-MSCs possess characteristic calcium [Ca2+] waves that are involved in intracellular signaling. Ca2+ oscillations have been found to play a key role in PEMF-induced cell differentiation to various tissue types [31]. The ability of PEMF to stimulate osteogenesis depends on the maturation stages of the osteoblasts where by increased expression of bone marker genes during differentiation and mineralization can enhance calcified matrix production [32,33]. The plasma membrane is often considered the main target for PEMF signals, and most results point to an effect on the rate of ion or ligand binding due to receptor sites acting as modulators of signaling cascades [16]. Electrical properties such as membrane surface charge and potential are especially influenced by extra-low frequency PEMF [34]. For example PEMF can induce depolarization in the cell membrane followed by an increase or decrease in intracellular calcium [Ca2+]i [34]. As a second messenger, Ca2+ ions are involved in regulation at all stages of cellular growth and development, including proliferation and differentiation, as well as in the assembling and disassembling of cytoskeletal elements [34].
What emerges from the considerable advances made in understanding the regulation of osteoprogenitor cells by growth factors is that there is extensive cross-talk between the signaling pathways activated by them [35]. This is also applicable to the interaction of PEMF and information transfer. The plasma membrane represents a significant barrier between the extracellular environment and the interior of the cell, both in terms of electrical resistance and in terms of information transfer. Exposure to exogenous stimuli such as a PEMF has been reported to promote proliferation and differentiation of BM-MSCs via ion dynamics and small signaling molecules [16]. The plasma membrane is often considered to be the main target for PEMF signals due to its effect on the rate of ion or ligand binding acting at the receptor site to modulate signaling cascades [16,36,37]. Ion fluxes are closely involved in the control of differentiation as stem cells move and grow in specific directions to form tissues. PEMF has been reported to affect numerous biological functions such as gene expression, cell fate, and cell differentiation; however, certain ranges of low-frequency amplitudes induce specific effects [16,37]. One plausible explanation is that PEMF may interact with already existing membrane signal-transduction mechanisms, which possess extremely high sensitivity and specificity for detecting and transducing low levels of signal in the extracellular environment [38].
In this study known osteogenic enhancing methods of combining PLLA scaffolds with MSCs were used to determine if PEMF could expedite the differentiation of bone for the purpose of treating osteoporosis. Previous studies have reported beneficial uses for MSCs for bone grafts because they can be easily isolated from adult bone marrow and differentiated naturally after trauma [15]. PEMF stimulation has been used for many years in the treatment of bone fracture healing, with clinical benefits [16,39] and several studies have demonstrated its capacity to increase bone tissue regeneration without adverse effects [13,40-42]. What is of particular interest is that the therapeutic parameters are most affective in the 15 Hz, [0.4 mT - 3.2 mT] range [16], so the effect appears to be frequency specific. PEMF has for many years been considered a promising alternative to drugbased therapies for osteoporosis by increasing bone mineral density and preventing bone loss [43,44]. PEMF can also be applied exogenously for a continuous effect post-op [45].The frequency and intensity the PEMF [15 Hz, 2.4 mT] used in this study is consistent with previous reports for MSC differentiation [16] and bone growth [46]. Further research is necessary to determine whether other osteogenic signals are being stimulated by the PEMF, and also whether sufficient vascularization is being produced to meet the growing tissue nutrient supply.
PEMF in combination with MSCs and BTE scaffolds can provide alternative treatments to the current problems of treating osteoporosis with pharmaceuticals, pathogen transfer due to autograft, and immune rejection due to allograft. Low-frequency PEMF has been reported to be effective in the enhancement of osteogenesis with no documented negative effects as reported in drug treatments and transplantation. Here we show the enhanced effect of a 15 Hz, 2.4 mT, for 20 min/day, 3x/week for 3 weeks. PEMF has the capability of stimulating bone growth in both osteoblasts and MSCs to form bone up to 30% faster that previously used methods. The window of efficacy for administration appears to be between 7-14 days. In summary, these results suggest that PEMF enhances the commitment of MSC seeded scaffolds to form osteoblasts more efficiently than differentiation media alone.
This work was supported by the Wake Forest Center for Integrative Medicine, grant no. 120-330-740196 and the Wake Forest Institute for Regenerative Medicine translational grant no. 730-120000-00000-110494. The author wishes to thank Graça Almeida-Porada, MD, PhD for her generous donation of the Stro-1 mesenchymal stromal cells [MSCs] used in this study.
The author has no conflicts of interest to report.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Ross CL (2017) Optimal Time of Efficacy for Using Bone Tissue Engineered Cell Therapies and Pulsed Electromagnetic Field [PEMF] for the Treatment of Osteoporosis. Cell Stem Cells Regen Med 3(1): doi http://dx.doi.org/10.16966/2472-6990.116
Copyright: © 2017 Ross CL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access