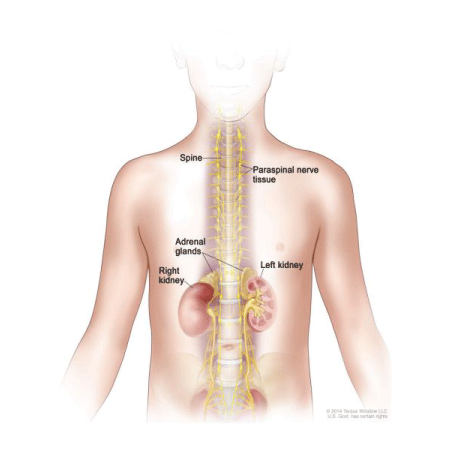
Figure 1: Neuroblastoma commonly develops in the adrenergic tissue of the adrenal glands or in the paraspinal nerve tissue that runs along the spinal cord [1].


Kailey Gorski Vincent S Gallicchio*
Department of Biological Sciences, College of Science, Clemson University, Clemson, South Carolina, USA*Corresponding author: Vincent S Gallicchio, Department of Biological Sciences, College of Science, 122 Long Hall Clemson, SC 29627, USA, E-mail: vsgall@clemson.edu
Neuroblastoma is a pediatric cancer that originates in neuroblasts. Because of the variability in patient responses to current treatment options, newer treatments are constantly being developed and tested in clinical trials. One such treatment option that has been showing a lot of promise in treating neuroblastoma is the use of allogeneic stem cell transplants combined with high dose chemotherapy, radiation and immunotherapy.
Neuroblastoma; Pediatric cancer; Clinical trials
Neuroblastoma is a type of cancer that develops in neuroblasts, which are immature nerve cells found in an embryo or fetus, and occurs most often in infants and children younger than 10 years old [1]. Although rare overall, this cancer accounts for around 50% of all cancers in infants, making it the most common solid tumor found in patients underthe age of one [1]. Five year survival rates for initially diagnosed neuroblastoma patients have a relatively wide range, spanning from 95% survival in patients under the age of 1 to 68% survival in patients between the ages of 1 and 14 [1].
Because neuroblastoma is a cancer of the sympathetic nervous system [2], the signs and symptoms of neuroblastoma can vary widely depending on the size of the tumor, the location of the tumor, if the tumor has metastasized, and if the tumor cells are capable of secreting hormones [3,4]. One of the most commonsymptoms associated with neuroblastoma is the development of tumors in the abdomen or pelvis. Tumor formation in this area may result in anorexia, (Figure 1) which could lead to unwanted weight loss or abdominal pain. Tumors in the abdomen may also cause edema in the legs if the tumor is blocking any lymph vessels that would prevent circulation of fluids in the body. Tumors can also form in the chest or neck. This may cause swelling in the face, neck or arms, headache, dizziness, and changes in consciousness if the cancer begins to affect the brain. After initial tumor formation, many patients also encounter the problem of metastasis.

Figure 1: Neuroblastoma commonly develops in the adrenergic tissue of the adrenal glands or in the paraspinal nerve tissue that runs along the spinal cord [1].
Typically in neuroblastoma patients, metastasis occurs in the lymph nodes and bones. If the cancer spreads to the lymph nodes, the patient may present with abnormal swollen lymph nodes across the body. If the tumor metastasizes into the bones, patients may complain of bone pain and may limp or refuse to walk altogether. If the cancer spreads into the bone marrow, hematopoiesis may be compromised and the child may be abnormally tired, weak, and prone to frequent secondary infections, or show excess bruising and bleeding [5]. Neuroblastoma may also present in patients as a variety of hormone-related symptoms, including constant diarrhea, fever, hypertension, tachycardia, and diaphoresis.These symptoms, causedby tumor secretedhormones, cytokines orpeptides, are often referred to as paraneoplastic syndromes [6].
Because sympathetic nerve cells release catecholamine’s, such as epinephrine and norepinephrine, into the blood, urine catecholamine tests are often performed on patients who are suspected of having neuroblastoma [1,7]. When catecholamines are metabolized, they are broken down into vanillylmandelic acid (VMA) and homovanillic acid (HVA) and are released into the urine. Increased levels of VMA and HVA may be indicative of neuroblastoma [8,9].
Imaging tests, such as ultrasounds, x-rays, CT scans, MRI scans, and MIBGscans may also be used as a diagnostic tool (Figure 2)in determining if a patient has neuroblastoma [1,10,11].They may be performed to detect the presence of a tumor, to learn how far the cancer has metastasized, and to determine if treatment has been effective in patients who have already been diagnosed.
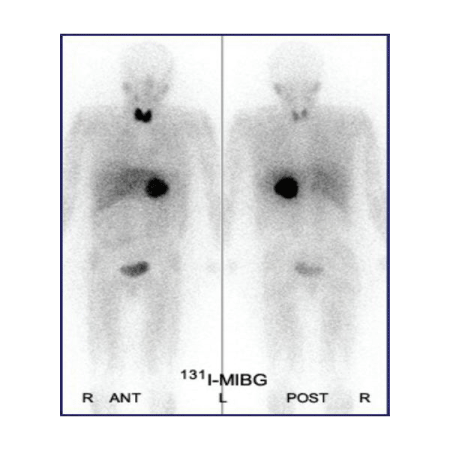
Figure 2: [6] 131I-MIBG scintigraphy of a neuroblastoma patient, showing tumor formation on the left adrenal gland.
Upon diagnosis, the stage of cancer will be identified to determine how far the cancer has metastasized in the body. The staging will affect the patient’s treatment, as well as their prognosis, and is determined by the results of physical exams, imaging tests, and biopsies of eitherthe primary tumor, or of surrounding tissues.
Many of the treatment options used for neuroblastoma are common among other cancer treatment options [12]. Typically, neuroblastoma is treated with surgery to debulk the tumor that is developing in the patient’s body, chemotherapy, commonly using combinations of cyclophosphamine or ifosamide, cisplatin or carboplatin, and vincristine among others, to destroy the malignant cells, and radiation therapy, which also targets malignant cells and works to destroy them [13,14]. Retinoid therapy may also be used in the treatment of neuroblastoma. This therapy option uses therapeutic differentiating agents that are related to vitamin A and are thought to help some cancerous cells develop into normal, mature cells [3]. Because neuroblastoma is a cancer of early nerve cells, inducing the cellsto mature through the use of therapeutic differentiating agentswould prevent them from proliferating into more cancerous cells. Neuroblastoma is also treated with immunotherapy, which uses monoclonal antibodies to help the patient’s own immune system recognize and destroy the cancer cells more effectively [15,1]. Currently, the most common monoclonal antibody used in thistreatment is dinutuximab (Unituxin) [16], which binds GD2, a type of ganglioside commonly expressed on the surface of neuroblastoma cells [17]. The final treatment option that is used in neuroblastoma patients is high-dose chemotherapy or radiation paired with autologous stem cell transplantation (ASCT) [18].
In order to determine the most effective treatments for neuroblastoma patients, clinical trials are performed to study whether some treatment regimens are more effective than others.
Most of the clinical trials involving stem cell transplants that have been done, and that are currently being researched, can be divided into 3 broad categories: radiation therapy followed by stem cell transplant, chemotherapy followed by stem cell transplant, and immunotherapy followed by stem cell transplant. The following clinical trials show a representative study from each of those categories.
One clinical trial, conducted by the Children’s Hospital Los Angeles and the National Cancer Institute from 2005 to 2016 (including other treatment locations across the United States and Canada) tested the effects of targeted combination chemotherapy and radiation therapy paired with ASCT in patients with relapsed or refractory neuroblastoma [19,20]. This was a phase 2 trial that studied how well administering 131I-MIBG with a combined regimen of chemotherapy could treat high-risk recurrent neuroblastoma patients, and to determine the maximum-tolerated dose (MTD) and toxicity of iodine-131-metaiodobenzylguanidine (131I-MIBG). 131I is a radioactive isotope of iodine. When introduced into the body, 131I is capable of stopping the growth of tumor cells and causing oncogenic tissue damage if it can be targeted to those specific areas of the body. When paired with metaiodobenzylguanidine (MIBG), the radioactive isotope is targeted directly towards the neuroblastoma cells specifically [21]. MIBG is a radiopharmaceutical that binds with the radioactive iodine and localizes to adrenergic tissue. Because the radiation therapy can be targeted so well to this tissue type, the use of 131I-MIBG is a fairly effective treatment for neuroblastoma patients [11,21-23].
In this clinical trial, a regimen of carboplatin, etoposide, and melphalan (CEM) were used as chemotherapeutic agents in combination with the 131I-MIBG therapy [19,20]. Prior to the treatment regimen, stem cells were collected from the patient through apheresis [24] and cryopreserved until ASCT. A minimum of 1.0 × 106 CD34+ cells/kg peripheral blood stem cells were required after collection for the patient to continue on with treatment [25,26].
The patient population tested in this trial consisted of high-risk neuroblastoma patients, ages 1-30 years, who had received no prior myeloablative therapies, and who showed uptake of MIBG prior to treatment [27]. Patients were divided into two cohorts based on their response levels to previous inductive treatments. Cohort 1 consisted of 42 patients that exhibited either no response to previous treatments or progressive disease after being diagnosed. Cohort 2 was comprised of 8 patients who showed progressive disease after completing at least 4 rounds of induction therapy [28].
Enrolled patients were administered intravenous infusions of 131I-MIBG followed by a 96-hour continuous infusion of CEM therapy 2 weeks following the infusion [29]. ASCT was completed 72 hours following the completion of chemotherapy. Filgrastim was given to patients along with the ASCT and was continued until their ANC was over 1500/uL for at least 3 consecutive days [29].
After the treatment stage of the trial was completed, the research team assessed the results 60 days post stem cell transplant and then again after 3 years since the start of treatment. At 60 days post stem cell transplant, both primary and secondary responses were recorded and after 3 years, only secondary responses to the treatment were assessed [8,20].
At 60 days post stem cell transplant, primary tumor responses to treatment were assessed using MIBG scintigraphy [30], CT/MRI imaging, urine catecholamine measurements and bone marrow aspirates or biopsies [20,28]. It was observed that 4 out of the 41 observable patients in Cohort 1 (1 of the patients originally enrolled in this cohort was not assessable due to surgical removable of his only observable soft tissue tumor prior to evaluation) exhibited a positive response to their treatment. Positive responses were identified based on reduction in tumor size and/ or decreased levels of neuroblastoma cells in the bone marrow. Of the 8 Cohort 2 patients, 3 showed a positive response to the treatments [20,28].
The secondary response assessed at 60 days post stem cell treatment was the engraftment dose-limiting toxicity (DLT) of the patients. This assessment measured the amount of either delayed stem cell engraftment in patients or failed stem cell engraftment. To measure this, blood cell counts were collected to see if there was an increase, decrease, or no change in the white blood cell and platelet productions from the newly introduced stem cells. Delayed and/or failed engraftment was defined as ANC<500/ μL after 28 days post ASCT, platelets<20,000/μL after 56 days post ASCT or if any additional stem cell infusions were required in the patient. Two of the Cohort 1 patients and 1 of the Cohort 2 patients showed failed engraftment [20,28].
The second secondary response measured 60 days post stem cell transplant was the diagnosis of veno occlusive disease (VOD) or sinusoidal obstruction syndrome (SOS). These disease symptoms can be characterized by hepatomegaly, hyperbilirubinemia, and other related conditions posttreatment [31,28]. After assessing the patients, it was found that 6 of the patients in cohort 1 developed VOD/SOS, and 0 of the cohort 2 patients developed VOD/SOS after receiving the high-dose chemotherapy and radiation therapy [32,20,28].
Three years since the start of treatment for the patients, event‐free survival (EFS) was estimated for the two cohorts. The EFS was described as the probably of the individual patients having no recurrence or increase in the disease presentation in the 3 consecutive years following the high-dose chemotherapy and radiation treatment regimen [20,33]. It was calculated that Cohort 1 patients would have a 20% ± 7% chance of being event-free after 3 years post treatment and the good‐risk patients would have a 38% ± 17% chance of being event free after 3 years post treatment [28].
This trial presented an overall effective treatment option for patients with high-risk neuroblastoma. The use of MIBG to deliver targeted doses of radiation therapy aided in not only effectively reducing tumor size and metastasis, but also allowed for lower total-body irradiation (TBI). Because MIBG targets at adrenergic tissues, patients experienced lower overall effects of the radiation in parts of the body that did not contain either primary or metastatic neuroblastoma [34], lowering the risk of late onset side effects commonly observed in patients who have undergone radiation therapy[19]. It was found that the rates of VOD and other toxicities related to treatment could not be directly caused by the use of this radiotherapy alone, and is consistent with the results of trials that utilize similar chemotherapeutic regimens. The use of ASCT allowed for supported engraftment after treatment, and therefore, aided in the reestablishment of hematopoietic tissue post-myeloablative therapy [19]. When compared to other similar trials, the amount of hematopoietic reconstruction observed in the patients of this clinical trial was essentially equal, showing that the addition of MIBG therapy to the treatment regimen had no additional negative effects on the bone marrow
Another clinical trial for the treatment of high-risk neuroblastoma patients was completed by the Department of Paediatric and Adolescent Oncology at the Gustave Roussy Cancer Campus in France [35]. This trial studied the effects of a high-dose chemotherapy regimen of thiotepa and busulfan-melphalan, followed by ASCT.
The 26 patients enrolled in this study had stage 4 neuroblastoma, were older than 1 year at the age of their diagnosis, and exhibited less than a partial response to prior treatments [35]. The first dose of the chemotherapy regimen included 300mg/m2 of thiotepa per dose, for 3 consecutive days. Oral busulfan was then administered in 4 equal doses for 4 consecutive days. A 140mg/m2 dose of melphalan was then given to the patients after a 24-hour time interval. Following each of the high-dose chemotherapy regimens, ASCT was performed, requiring 6 × 106 CD34+ cells/kg [35]. At the end of the treatment, the progress of the neuroblastoma in each of the patients was assessed based on tumor response via CT scans, MRI scans or MIBG scans. The researchers conducting the study also collected 2 biopsies of tumor tissue and 4 aspirates of the patient’s bone marrow in order to evaluate the morphology of the hematopoietic tissue [35]. The results of these tests were used to determine the event-free survival and the overall survival of the patients in the 3 years following treatment.
Prior to determining the event-free survival and overall survival, tumor responses in the patients were assessed. After receiving high-dose thiotepa therapy, it was observed that 4 of the total 26 patients enrolled in the study were in complete recovery, 12 were in partial recovery, 6 patients had stable disease and 2 of the patients were not evaluated. In this study, complete responses were defined as cases where there was a decrease in the metastatic sites in the patient, partial response was a decrease in less than 50% of the number of metastatic sites, and stable disease was no decrease in the number of metastatic sites, but also no further metastasis of the disease [35]. The 2 patients that were not evaluated developed progressive disease and therefore did not show a positive response to the treatment regimen.
The status post-high dose thiotepa chemotherapy was similar across all patients in the trial. All patients that received the treatment experienced grade 4 myelo suppression with neutropenia and thrombocytopenia, resulting in a need for supportive care in the form of platelet and/or RBC transfusions. In addition, 8 patients experienced mucositis and 4 patients experienced diarrhea. There was no reported toxicity-related death in patients treated with high-dose thiotepa [35]. Post-high dose busulfan-melphalan treatment, patients also experienced some related toxicity. Neutropenia and thrombocytopenia were, once again, observed in all participating patients. Unlike the mainly gastrointestinal problems associated with high- dose thiotepa treatment, SOS was the main toxicity associated with high-dose busulfan- melphalan treatment. SOS was observed in 11 out of the 24 patients, and there was only 1 related death [35].
This clinical trial presented another effective treatment option for patients with high-risk neuroblastoma. Compared with studies not involving high-dose chemotherapeutic regimens, this trial resulted in significantly higher rates of 5 year EFS (30 ± 4% vs. 19 ± 3%). The death rates associated with this trial were also lower than the accepted international protocols for high-risk neuroblastoma [35]. Although higher doses of chemotherapeutic agents were administered than have been historically administered and therefore, patients may be at a higher risk of toxicity effects, the high-dose regimen presented in this trial was better able to treat the metastatic and refractory disease originally observed in the patients better than the common dosage of the chemotherapeutic agents.
The Children’s Oncology Group conducted a phase 3 trial investigating the differences in neuroblastoma patient outcomes when administered isotretinoin alone or isotretinoin with dinutuximab, aldesleukin, and sargramostin following autologous stem cell transplant [36,37].
This study was primarily focused on determining the event-free survival after treatment, comparing the differences in outcomes between the two treatment groups [37]. The patients enrolled in this study were under 31 years of age and were diagnosed with high-risk neuroblastoma or developed high-risk neuroblastoma from a non-high-risk previous diagnosis.
Once entered into the trial, patients, who had shown some response to induction therapies and stem cell transplantation, were assigned to one of two experimental treatment groups. The first group was the standard treatment group and was administered 6 cycles of isotretinoin therapy [38]. The second group was the immunotherapy group, and was administered 6 cycles of isotretinoin along with 5 cycles of the ch14.18 monoclonal antibody, with alternating schedules of GM-CSF and IL-2 [37].
In the comparison of event free survival after 2 years between the two treatment groups, it was found that 46 ± 5% of the patients that were treated with isotretinoin with stem cell transplant (standard treatment group) and 66 ± 5% of patients treated with isotretinoin, dinutuximab, aldesleukin, and sargramostim with stem cell transplant (immunotherapy group) showed a 2-year event-free survival. These results showed that immunotherapy [39], in combination with stem cell transplantation, are associated with a significantly better patient outcome when compared with standard treatments in patients with high-risk neuroblastoma [37]. As a result, the study stopped randomization of treatments based on the significantly better outcomes observed in the immunotherapy group. In addition to increased EFS, the immunotherapy group also showed higher overall survival rates compared to the rates of the standard therapy regimen42. Most of the toxic effects observed in this trial were abdominal pain, hypotension, capillary leak syndrome associated with treatment cycles involving IL-2, and hypersensitivity reactions that were closely associated with the treatment cycles involving IL-2 as compared to the cycles involving GM-CSF [37]. There was only one treatment-related death that was attributed to a medication error, and most of the toxic effects that were observed stopped following the end of treatment. The purpose of including an immunotherapeutic regimen to the treatment cycle after ASCT was to increase the amount of antibody-dependent cell-mediated cytotoxicity of tumor cells expressing GD2, a function that is reduced in a majority of neuroblastoma patients. Upon completion of the trial, it was found that the cases involving such immunotherapeutic agents reported significantly higher rates of both EFS and overall survival. While the 2-year EFS of 66% of patients in the immunotherapy group is markedly better than the 2-year EFS of patients in the standard therapy group, it is important to note that patients diagnosed with recurrent, high-risk neuroblastoma, as in this trial, are not commonly fully cured of disease post-treatment [37].
The results of these clinical trials, along with many other clinical trials utilizing ASCT after high-dose chemotherapy, radiation therapy, or immunotherapy show that the use of 131I-MIBG paired with a high-dose CEM chemotherapy regimen, a high-dose chemotherapy regimen followed by ASCT, or immunotherapy paired with stem cell transplantation works at targeting tumors in refractory neuroblastoma patients [26]. The rates of patient responses reported in these trials were relatively low compared to the rates for other common cancers, but considering the refractory nature of the patient populations that were studied and comparing those rates to other treatment regimens that are commonly used for refractory neuroblastoma, these treatments fared favorably.
Although the results of the trials were not all significant when compared to more commonly used treatment regimens, the clinical trials did not present a bad or failed treatment option to the patients enrolled in them. Rather, the treatments used in these clinical trials didn’t increase the patient’s chance of survival any more than another treatment regimen would have.
It is also important to consider that the patients that were enrolled in these studies all had relapsed or refractory neuroblastoma. Having a relapse in this disease specifically makes treatment much more difficult [40]. The tumors often show a much lower response to typical treatment regimens, and therefore, the patient’s chance of survival is drastically lowered compared to the chances of survival after being first diagnosed with neuroblastoma. The fact that the patients showed some positive response to the newly investigated treatment regimens is a great improvement and advance in the management of this cancer.
There are no real controversies associated with the use of stem cells for the treatment of neuroblastoma. The stem cells used in these trials, as well as other treatment options that have been well established to treat neuroblastoma, are not embryonically derived, which is where most of the controversy concerning stem cell research typically comes from. Since the treatments also use an autologous transplant, there are no real complications with immune rejections of the stem cells after receiving the transplant. In allogeneic transplants, even though donors are matched to the stem cell recipient, there is always the possibility that the recipient’s immune system will recognize the stem cells as foreign and attack them in an immune response [19,41]. This immune response, in reaction to rejecting the newly transplanted stem cells, could cause various complications for the patient and may even cause death if the immune response becomes systemic and puts the patient in a state of shock.
Overall, the treatment regimens investigated in the clinical trials conducted by the Children’s Hospital Los Angeles and the National Cancer Institute, the Department of Paediatric and Adolescent Oncology at the Gustav Roussy Cancer Campus, and the Children’s Oncology Group provide some hope for the targeted treatment of recurrent neuroblastoma patients.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Gorski K, Gallicchio VS (2017) Autologous Stem Cell Treatments for High Risk Neuroblastoma. Cell Stem Cells Regen Med 3(1): doi http://dx.doi. org/10.16966/2472-6990.115
Copyright:© 2017 Gallicchio VS et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access