Abstract
Disseminated tuberculosis is a form of widespread bacilli infection with typical involvement of the lungs and other extra pulmonary organs. It
is a rare manifestation of mycobacterial infection and unusual among immune competent individuals.
We present 2 cases of disseminated Mycobacterium tuberculosis infection in two otherwise healthy adults: the first is a 62-year-old man,
heavy smoker and with alcohol abuse, who was admitted to our hospital in February 2014. He was being treated for multi sensitive disseminated
tuberculosis (lung, lymphatic, vertebral, musculoskeletal, and testicular involvement) since March 2013 with a combination of classic antituberculous
agents (isoniazid and rifampicin; had stopped pyrazinamide and ethambutol in August 2013) and was admitted due to vertebral
tuberculosis with epidural empyema associated with multiple para vertebral and retroperitoneal tuberculous abscesses, failing to respond to the
initial conventional therapy and requiring surgical drainage.
The second refers to a 27-year-old man with no previously known medical disorders, referred to our center in early June 2015 with non-specific
constitutional symptoms and acute dry cough, and was diagnosed with disseminated tuberculosis with non-cavitary nodular lung involvement,
cervical, supraclavicular, mediastinal and retroperitoneal lymphadenitis, pleural and peritoneal effusion and hepatosplenomegaly. A cervical
lymph node biopsy was performed, revealing caseating granulomas, with a positive DNA probe followed by confirmatory cultural study for multi
sensitive Mycobacterium tuberculosis. The patient is now being treated with a conventional four-drug regimen.
No primary or secondary immunodeficiency was diagnosed following a thorough study during the investigation of these two patients.
Disseminated tuberculosis is a rare and potentially lethal form of tuberculosis that usually affects immune compromised patients. The infection
of healthy adults and the occurrence of extra pulmonary involvement is a diagnostic challenge and its poor prognosis can be outfought with the
early initiation of anti tuberculous agents and appropriate surgical therapy, when prompted.
Keywords
Disseminated tuberculosis; Widespread infection; Healthy adults; Potentially lethal; Anti tuberculous agents
Introduction
Disseminated Tuberculosis (TB) is a rare form of TB infection
characterized by the lymphatic and hematogenous spread of Mycobacterium
tuberculosis bacilli. Although the primary site of infection is usually the
lung, other organs may be involved either in primary or post-primary
infection [1]. In healthy individuals, this dissemination is contained by
a prompt immune response, particularly cell-mediated immunity and
the interferon (IFN) γ pathways, limiting the infection [2]. Therefore,
clinical disseminated disease following primary or reactivation of
established latent tuberculosis is more often in relatively or absolutely
compromised hosts, such as infants, patients with Acquired Immune
Deficiency Syndrome (AIDS) or patients with latent infection placed
on a tumor necrosis factor alpha blocker, compromising the regular
immune surveillance [3,1].
Rarely, TB dissemination occurs in healthy individuals, with no
known pre-disposable condition, challenging our clinical suspicion to
consider it as a differential diagnosis, especially in the setting of the
non-specific clinical features of this form of disease, such as the ones
presented in this report.
Case Report 1
A 62-year-old man, with alcohol abuse and heavy smoker with no
know lung disease or history of past infections was being treated for
disseminated TB since March 2013. He had been admitted in another
hospital and had a report referring multiorgan involvement including
pulmonary, lymphatic, testicular, vertebral and paravertebral with
bilateral abscesses by a multi sensitive Mycobacterium tuberculosis, and
was being treated with a combination of classic anti-tuberculous agents
(isoniazid [INH], rifampicin [RIF], pyrazinamide [PZA] and ethambutol
[EMB], having stopped the last two after 16 weeks of therapy) with
Directly Observed Therapy (DOT). He was Human Immunodeficiency
Virus (HIV) seronegative and was on oral prednisolone (20 mg/ day) on
the beginning of treatment.
He had chronic lower back pain following the vertebral and paravertebral
involvement and was referred to our center in February 2015 following
worsening symptoms in the past couple of months, followed by an MRI
performed in ambulatory that revealed spondylodiscitis involving the
posterior from D12 to L1 and L3-L4 with an anterior spinal epidural
empyema along the D9-L4 vertebrae with spinal cord deformation (Figure
1). The MRI also showed multiple bilateral paravertebral abscesses
alongside the psoas major muscles, with a massive 130 mm formation,
on the left side.
Initial physical exam revealed vital signs within normal limits and
oxygen saturation of 97% on ambient air. He had pale mucous membranes
and thoracic and abdominal examinations were unremarkable. He
presented with a normal mental status and showed no signs of medullar
compression whatsoever. Laboratory test showed normal White Blood
Cell (WBC) population count; hemoglobin was 10.0 g/dL, with normal
hepatic transaminases, bilirubin and renal function. The chest CT scan
revealed a 43 mm wide cavitary lesion with an air-fluid level in the lower
lobe apical segment of the left lung associated with disperse bilateral small
nodular opacities, suggesting a miliary pattern (Figure 2).
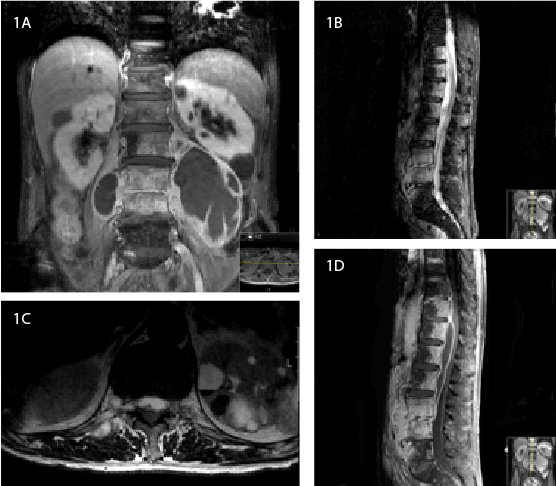
Figure 1: MRI study. 1A) T1 weighted coronal view showing massive
paravertebral abscesses, alongside psoas major muscles, as well as
enhanced disc, perivertebral soft tissues and vertebral bodies, mainly L3-
L4 and L4-L5; 1B) Sagittal T2-STIR view with a spinal epidural collection
ranging from D10 to L4; 1C) T2 weighted axial view showing medullary
cone compression at D12-L1 level; 1D) Saggital T1 weighted exhibiting
medullary cone compression.
He was submitted to percutaneous drainage of the left psoas major
abscess, with a positive acid-fast smear and molecular assay with
polymerase chain reaction (PCR) for Mycobacterium tuberculosis,
although no bacilli were cultured on Lowenstein-Jensen or Middle brook
growth mediums. Initial quadruple therapy was maintained with INH,
RIF, PZA and EMB.
Failing to achieve clinical improvement, amikacin, moxifloxacin,
ethionamide, cycloserin and para-aminosalicylic acid were added to INH,
RIF and EMB on the founded clinical suspicion of drug-resistant TB and
the patient underwent successful surgical drainage of the paravertebral
abscesses on day 20.
Faint clinical progress was followed by retrosternal chest pain with
electrocardiographic and echocardiographic evidence of acute pericarditis
with moderate pericardial effusion. A non-steroidal anti-inflammatory
drug was initiated with a favorable clinical course, but mild pericardial
thickening developed and 1 mg/Kg/day of prednisolone was introduced
to avoid progression to constrictive pericarditis.
Expressive aphasia and right-sided hemi paresis suddenly developed
on the 28th day of hospitalization. The cranial CT scan showed just a
cortical hyperdense multifocal lesion on
the left frontal, temporal and parietal lobes, suggestive of bacilar
tuberculomas, as well as vascular narrowing of the supraclinoid segment
of the left internal carotid artery, involving the sphenoidal segment of
the middle cerebral artery, producing hipodense lesions in the left lateral
post-central gyrus and the frontal semiovale center (Figure 3). These
findings alluded ischemic changes due to infectious vasculitis, albeit
the patient was already on systemic corticosteroids, meanwhile changed
to dexamethasone. A lumbar puncture was performed and meningitis
was excluded. A bronchoalveolar lavage was performed, confirming a
positive PCR and acid-fast stain for TB. Despite no growth was obtained
on cultural studies, molecular assays revealed susceptibility to first-line
therapy drugs, INH and RIF, prompting an investigation of alternative
causes of treatment failure. He was confirmed to be HIV seronegative, as
well as Human T Lymphotropic Vírus (HTLV) 1 and 2. Immunological
tests showed normal immunoglobulins and no specific antibodies were
detected, including anti-IFN γ, and no production deficit was detected
in this interferon. The patient later developed a severe lymphopenia, with
no selective deficiency detected in lymphocyte subsets and no IgG or IgM
anti-lymphocyte antibodies were reported. Further investigation revealed
no CD119 deficiency and no endoscopic or radiographic findings were
suggestive of tuberculous enteritis, confirmed by a normal histology and
culture-negative tissue.
Based on the molecular susceptibility pattern, conventional INH,
RIF, PZA and EMB therapy was resumed with progressive clinical
improvement, elicited by pain amelioration on analgesic drugs,
sustained increase in lymphocyte count achieving normal range values
and gradual radiologic recovery (Figure 4). Right-sided hemi paresis
and motor aphasia persisted and the patient was discharged conducting
a rehabilitation program in a convalescence unit, 135 days after he was
admitted, on DOT with INH, RIF, PZA, EMB and dexamethasone.
Subsequent outpatient management would be guaranteed in the local
tuberculosis diagnosis and treatment center.
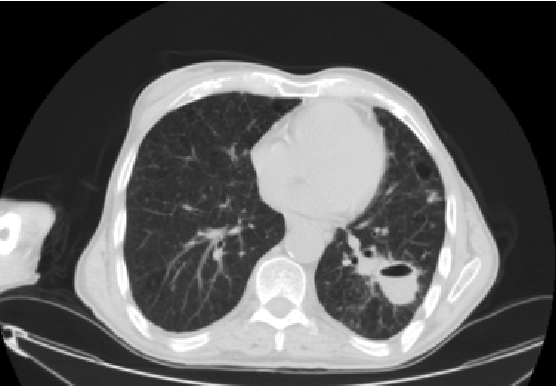
Figure 2: Chest CT scan of the cavitary lesion in the apex of the left
lower lobe.
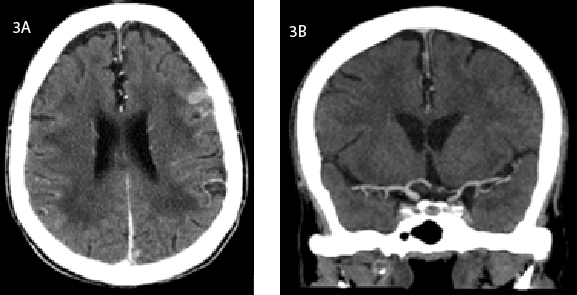
Figure 3: A) Cerebral tuberculoma on an axial view of the brain CT scan;
B) Contraste enhanced coronal view showing diffuse narrowing and
irregularity of the supraclinoid segment of the left internal carotid artery.
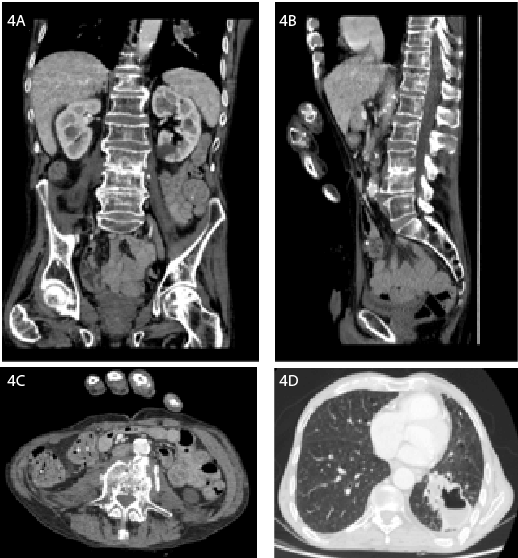
Figure 4: A, B and C. Coronal, sagittal and axial scans of the abdomen
and pelvis showing non-enhanced residual lesions in the paravertebral
regions and alongside the psoas major muscles, greatly improved when
compared to initial MRI images; D. Larger volume cavitary lung lesion but
peripherally non-contrast enhanced, suggesting abscess in resolution.
Case Report 2
A 27-year-old, previously healthy man was referred to our hospital
in May 2015. He had presented with a constitutional syndrome that
developed in the past month, which included asthenia, anorexia and
weight loss of 15% of body mass index. Two weeks before admission he
started complaining of an unproductive cough and pleuritic chest pain
on the lower right hemi thorax. His family physician performed him a
chest-abdomen-pelvis CT scan that revealed bilateral apical fibrous scars
of the lungs, an imprecise solid nodular area in the right apex, small rightsided
pleural effusion, upper Para-aortic lymphadenopathy and small
peritoneal effusion. He had been vaccinated for as a child, had no history
of symptomatic primary tuberculosis, known contact with infected
individuals or recent travel history and was admitted for investigation.
Initial physical examination exhibited normal vital signs and oxygen
saturation on ambient air. Non painful right-sided enlarged lateral cervical
and supraclavicular lymph nodes were palpable, with a maximum diameter
of 20 mm, with a non-adherent elastic consistency. No abnormality was
detected on chest and abdomen exploration and no other lymph nodes
were apparent.
Basic laboratory tests included a normal WBC count and hemoglobin,
renal function, transaminases and bilirubin levels.
A CT scan of the chest, abdomen and pelvis reported the previous
fibrotic changes and nodular area in the right upper lobe, added to upper
left lobe emphysema with no signs of pleural or pericardial effusion, in
addition to numerous conglomerate mediastinal and retroperitoneal
lymph nodes with signs of central necrosis, the largest measuring 32
mm (Figure 5). Mild enlargement of the spleen, liver and pronounced
densification of the peritoneum was also observed, with no peritoneal
effusion.
Mandatory study included negative consecutive acid-fast stains;
Lowenstein-Jensen and Middle brook culture growth mediums of the
sputum, followed by a positive Quantiferon-TB and a bronchoalveolar
lavage revealing positive acid-fast stain for
Mycobacterium tuberculosis along with cultured bacilli. Excisional
cervical lymph node biopsy for histopathologic and microbiologic
evaluations exhibited necrotizing granulomatous lymphadenitis, positive
molecular assay and cultural growth of a drug-susceptible strain of
Mycobacterium tuberculosis.
The patient was confirmed to be HIV seronegative and the following
workup for underlying immunodeficiency was negative and included
normal immunoglobulin levels, lymphocyte subsets, and specific
antibodies.
A conventional 4-drug regimen with INH, RIF, PZA and EMB was
initiated and he was discharged for outpatient management.
Discussion
Disseminated tuberculosis is defined as the widespread form of
Mycobacterium infection mostly involving the lungs, lymph nodes, liver,
pleura, pericardium, spleen, meninges and bone [4]. It is highly uncommon
and it is estimated that disseminated TB accounts for 1 to 2% of all cases of TB
infection in immune competent individuals [5]. 349 cases were reported in
the United States in 2012, a minor increase of 1.8% since 2006 [6].
Commonly known conditions favor extended bacillary disease, such
as malnutrition, advanced age, pregnancy, alcohol abuse, corticosteroid
or other immunosuppressive and cytotoxic therapy including biologic
agents, connective tissue diseases, diabetes mellitus, malignancy, organ
transplantation, renal failure and most notably HIV infection, where
miliary infection accounts for nearly 10% of all cases [3,7-10]. Thus, a
thorough investigation for primary or secondary immune deficiency is
imperative, as it can change the therapeutic approach and global outcome.
Classic presentation is variable and generally includes a sub acute
or chronic constitutional syndrome with other absent symptoms or
reflecting the underlying organ involved. Can also have a wide-ranging
clinical picture, from nocturnal hyperhidrosis, fever of unknown origin
or even multi organic failure and, therefore, should always be considered
even in the immune competent adult [3].
In general, antimicrobial therapy for disseminated tuberculosis is the
same as pulmonary infection although modifications may be warranted
in the setting of drug-resistant TB and longer duration of therapy may be
appropriate for high organism burden or slow clinical response, immune
suppression, CNS infection and in certain patients with bone and joint
involvement, where a surgical approach is sometimes mandatory [11,12].
Despite adequate therapy for a drug-susceptible TB strain in the first
patient, musculoskeletal lesions failed to improve and he progressed
with acute pericarditis and pericardial effusion followed by Central
Nervous System (CNS) vasculitis, determining a meticulous immune
status workup. Unaffirmative results emerged other questions, such as the
insufficient (CNS) penetration of certain anti-tuberculous drugs, like RIF
or EMB when compared to moxifloxacin or ethionamide, illustrating the
adverse neurological result in this case [13,14].
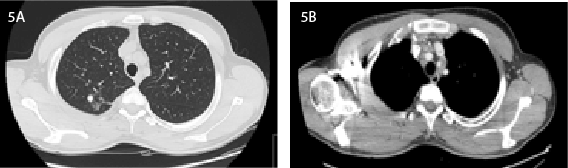
Figure 5:Chest CT scan showing a (A) nodular area in the right upper
lobe and (B) numerous mediastinal conglomerates with central necrosis.
Another explanation would be the development of a paradoxical
Tuberculosis-associated Immune Reconstitution Inflammatory Syndrome
(TB-IRIS), with the patient developing signs of disease progression after
initial response to treatment. These reactions, largely described in HIV
infection, also occur in seronegative individuals and manifestations
include recurrent fever, worsening pulmonary infiltrates, pleural or
pericardial effusion, new or enlarging tuberculomas and CNS invasion.
Pathogenesis of TB-IRIS accounts for the release of new antigen targets
during mycobacterial elimination, hypersensitivity to those antigens
and excessive immune restoration following bacillary-induced immune
suppression in the initial treatment phase [15]. No such workup or
hypotheses were conducted for the second patient, as there were no
evidences of treatment failure.
Lymphopenia stands for one of the most common laboratory
abnormalities in TB-infected patients, in addition to anemia, leucopenia,
thrombocytopenia, erythrocyte sedimentation rate, C-reactive protein,
hyponatremia or hypocalcaemia, and it constitutes an independent risk
factor of mortality [16,17]. It installed progressively in the first patient
and prompted an immediate check for underlying causes, which failed
to provide a consistent unequivocal response. Considering lymphocyte
recovery during anti-tuberculous drug therapy, it was presumed to be
secondary to infection via redistribution in secondary lymphoid organs.
Adjunctive systemic glucocorticoid therapy is recommended for
treatment of TB involving the CNS and pericardium but data is limited
and based on small case reports and scant clinical series [4,18,19]. Both
forms occurred in the first patient, encouraging steroid therapy, resulting
in pericarditis resolution but failed to achieve neurologic recovery, in
accordance with the expected morbidity described in literature and
establishing it as an independent risk factor of mortality in most studies
[20]. Other predictors are pancytopenia or lymphopenia, age, late
presentation and serious underlying disease [16,21,22].
Conclusion
Disseminated tuberculosis is a rare, devastating and potentially fatal
form of tuberculosis. It arises from lymphohematogenous spread of the
bacilli often resulting in a clinically inapparent condition, but encloses a
high morbidity and mortality burden, prompting a high index of suspicion
and requiring swift therapeutical action.
Albeit more common in the immune deficient patient, it can also
affect healthy individuals, offering a challenging diagnosis in the path of a
meticulous investigation.






