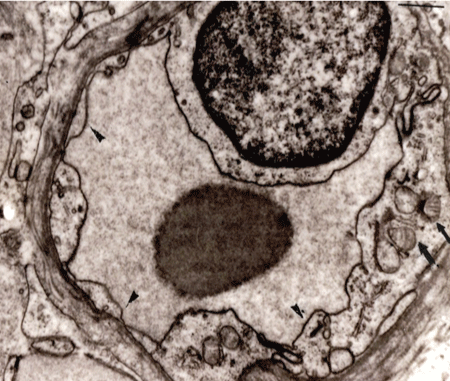
Figure 1: Normal capillary vessel of the bronchus. The plasma membrane (arrowheads) is observed clearly.


Ichiro Mochizuki1* Takayuki Honda2 Masayuki Hanaoka1
1Department of Internal Medicine, Shinshu University School of Medicine, Japan*Corresponding author: Mochizuki I, Department of Internal Medicine, Shinshu University School of Medicine, Japan, Tel: 81.263.33.7215; E-mail: p-ichiro@sea.plala.or.jp
The plasma membrane of the capillary endothelial cells showed that the both a little unclear and unclear or disappeared is 89% (34/38 cases) and control cases 32% (8/25) in the broncho-alveolar tract in the patients with sarcoidosis at the electron microscopic level. The clear plasma membrane in sarcoidosis is 11% (4/38) and control 68% (17/25). Early sarcoidosis cases showed a wide area of defective plasma membrane of the capillary endothelial cells.
Realization of the biphasic, crescent figured lipid droplets involving unsaturated fatty acid and lysosomal granules are 38% in frequencies and specific findings for sarcoidosis patients and having no lipid droplets in controls. Dark monophasic (unsaturated) lipid droplets with lysosome showed 25% in sarcoidosis patients, and 0% in controls. We demonstrated that lipid substances originated of swollen mitochondria, we suggested that the above described endothelial plasma membrane defect was mostly responsible for genesis of the lipid droplets with lysosome granules.
About findings of the autonomic nerve fibers surrounding capillaries in the main bronchus in sarcoidosis are observed swelling, elongation of myelinated fibers, and demyelinaton. Interestingly, we could to see the same biphasic, crescent figured lipid droplets in the peripheral nerve fibers. This fact suggested that the sources of the lipid droplets are originated from not only the capillary endothelial cell membrane of the respiratory tract, but also broadly capillary endothelial cell in the peripheral nerve in myelin. Our immunohistochemical study in von Willebrand factor 11 exactly suggested that sarcoidosis is disease of the capillaries in meaning of vast extent.
PLA2 as a target enzyme act on the membrane phospholipids and degrade the capillary wall, but the changed lysophpspholipids protect oneself against infection, and behave immunosuppression. Stomatin and stomatin-like proteins (SLP-2) locating at the plasma membrane interact with lipid droplets suggesting a role in intracellular lipid transport and suggest important membrane organizing function. Furthermore, SLP-2 associated with the plasma membrane and T cells and an important player in T cell activation, in which sarcoidosis granuloma formation depends on T cell modulation.
In conclusively, we detected defect in the plasma membrane of the broncho-alveolar capillary at the ultrastructural level, and following lipid metabolic anomaly introduce a critical change by PLA2 and SLP-2, in which situated in the plasma membrane in sarcoidosis.
Plasma membrane; Mitochondria; Lipid droplets with unsaturated fatty acids; Autonomic nerve fibers
Among less activity of the further profound research work of sarcoidosis with unknown etiology, amazing progress in studying about the superfamily of phospholipase A2 (PLA2) [1] enzyme catalyzing the hydrolysis of membrane glycerophospholipids and stomatin-like protein 2 (SLP-2) [2,3] an expressed main mitochondrial protein seems to bring a enlightening effects to people that continued to study abnormal lipid metabolism in sarcoidosis. For sarcoidosis study, there were some papers involved of lipid metabolism [4-10] but they have not touched the very core of a subject in pathogenesis. We have studied about fine structure of the capillary endothelial cells in broncho-alveolar tract in sarcoidosis patients [6-12] and recently we found the findings of plasmamembrane defect in the capillary endothelial cells in sarcoidosis, in which PLA2 enzyme exist in the same endothelial cells [1]. Changes of phospholipid membrane in the endothelial cells seems to exert an influence upon mitochondria in the same endothelial cells, and the changes of the myelinated nerve cells around the capillary endothelial cells in sarcoidosis [12] seems to be understandable in interpretation by PLA2 . The purpose of this paper is a wide extent of changes on plasma membrane of the capillary endothelial cells including in lipid substances and relationship between the lipid droplets involving polyunsaturated fatty acid and their origin in mitochondria or plasma membrane. We discussed on stomatin and stomatin-like proteins (SLP2) localizing in the plasma membrane and interact with lipid droplets.
Thirty eight transbronchial biopsy, twenty five transbronchial lung biopsy and two open lung biopsy specimens consisted of 35 males (mean age: 30 years; range 16-63)and 30 females (mean age: 38 years; range 15-60), and included 5 patients with Stage 0 (no lung involvement), 38 patients with Stage I (hilar enlargement alone), 20 patients with Stage II (hilar enlargement plus interstitial lung disease), 1 patients with Stage III (interstitial lung disease alone) (Scadding, 1961). The control specimens were obtained by transbronchial biopsy in 41 patients. They include 7 with lung cancer, 2 with long progressed miliary tuberculosis, 4 with old lung tuberculosis and others. The tissue classification of the lung cancer patients didn’t do, but the all cases were studied about lipid droplets in electron microscopic photographs [9].
Lung tissues were immediately cut into small pieces. The samples were fixed in 2.5% glutaraldehyde for 24 hours ethanol series, and embedded in Epon 812. Ultra thin sections were examined using a Hitachi 8 (Hitachi High-Technologies Corp. Tokyo, Japan), Hitachi 9 (Hitachi High-Technologies Corp.) and JEM 1200EX II (Jeol Ltd., Herts UK) electron microscope.
About a finding of the plasma membrane covering the capillary endothelial cells, were classified into three categories (clear, a little unclear and unclear or disappear) in each case. In each specimen, the founded lipid droplets were all recorded and morphologically divided into three groups and regard the subject with high frequencies in three groups as candidate.
About lipid droplets in the submucosal tissue of the capillary endothelium, morphologically classified and described various figures including lamellar structure by time or disease process. Autonomic nerve fibers in the bronchus and lung tissue in sarcoidosis were examined and described abnormal myelinated and amyelinated fibers.
Vascular endothelial cells, embryologically derived from mesoderm have a common progenitor with blood cells involving immune cells, and the capillary endothelial cells though they are flat cells show an important multitalent behavior in inflammation (Figure 1). The plasma membrane, covering with endothelial cells are consisted from lipo-protein components might have critical information about disease pathophysiology. Table 1 shows the plasma membrane of capillary endothelial cells in sarcoidosis patients. The findings of both a little unclear and unclear or disappearance reached to 87% in frequencies and controlness shows 28.1%.

Figure 1: Normal capillary vessel of the bronchus. The plasma membrane (arrowheads) is observed clearly.
| Sarcoidosis (38 Cases) | Controls (25 Cases) | |
| Clear | 4(11%) | 17(68%) |
| A Little unclear | 16(42%) | 2(28%) |
| Unclear or Disappear | 18(47%) | 1(0.1%) |
Table 1: The plasma membrane of the capillary endothelial cells in sarcoidosis patients.
As figure 2 shows, in early stage of sarcoidosis patients, the endothelial cells protruded into the lumen and we have found entirely defecting plasma membrane in whole extent in which this final result of abnormal metabolism has connection with significant thickening of this basement membrane, and increase of the dilated smooth endoplasmic reticulum causing activation of protein metabolism. Also morphological change showing appearance of the lipid droplets means abnormal lipid metabolism. Figure 3 show that there was a local deficit of the plasma membrane attached with blood platelets or eosinophils in the sarcoidosis patients. Adhesion with blood platelets with defecting plasma membrane showed 73% in 33 cases with sarcoidosis patients and 17% in 54 control cases. This finding shows that coagulation effect in the destroyed endothelial cell. In right side of figure 3, the local deficit of the plasma membrane and increase of Weibel-Palade bodies storing up the von Willebrand factor playing a role as coagulation system. Four figures in figure 4 are separate cases and not same ones of sarcoidosis patients. But these findings are very similar in shape of lipid droplets showing two kinds of special crescent-shaped dark color and lucent area. The latter seems neutral fat.
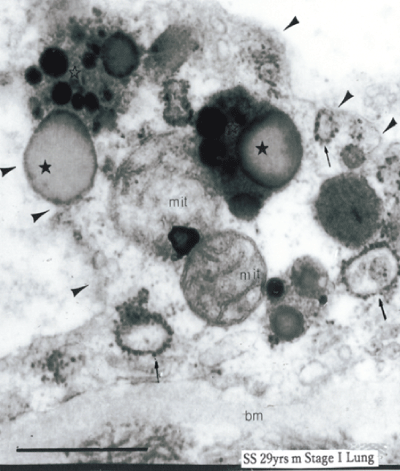
Figure 2: The defective plasma membrane of a active sarcoidosis case (operated lung specimen) (arrowheads). The protruded endothelial cell has no clear plasma membrane and basement (bm) is conspicuously thicked. Mitochondria (mit) are swelled and surrounded by dark monophonic lipid droplets (asterisk) with lysosomes. Increased dilated smooth endoplasmic reticulum (arrows) showed activated protein metabolism.
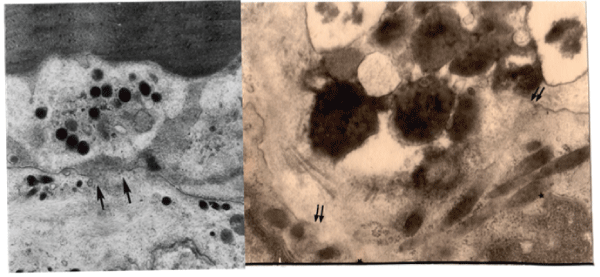
Figure 3: The local defecting plasma membrane (between arrows) and attaching with the blood platelet (left) and the eosinophil (right) attaching at the same place of the defecting plasma membrane (between double arrows). Rod body structures in the cytoplasm of the endothelial cell show Weibel-Palade bodies, having von Willebrand factor playing coagulating role.
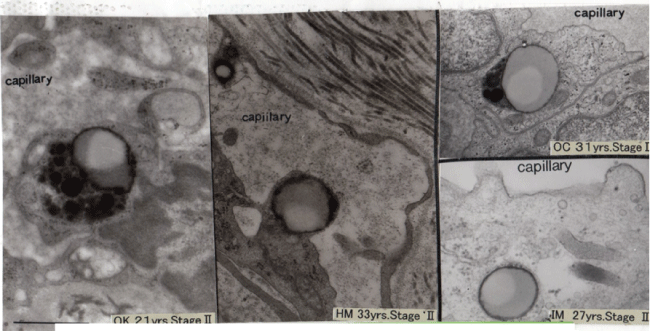
Figure 4: Four separate sarcoid cases together show the biphasic lipid droplets at the same capillary endothelial cells.
Table 2 shows the three types of classification about the lipid droplets in the capillary endothelial cells of the broncho-alveolar tract in the patients with sarcoidosis. Dark monophasic only lipid droplets are 35% (9/26 cases) and biphasic lipid droplets involving crescentshape are 38% (10/26 cases), and together73% in cases are contained an important dark, unsaturated fatty acid substances without in control cases. Lucent monophasic lipid droplets show 27% (7/26 cases) and control cases 18% (2/11 cases). One control case is a miliary tuberculosis and another lung cancer.
| Sarcoidosis (26 Cases) | Controls (11 Cases) | |
| Dark monophasic lipid droplets | 9 (35%) | 0 (0%) |
| Biphasic lipid droplets | 10 (38%) | 0 (0%) |
| Lucid monophasic lipid droplets | 7 (27%) | 2 (18%) |
Table 2: Three types of lipid droplets in the capillary endothelial cells of the broncho-alveolar tract in the patients with sarcoidosis.
Figure 5 show lipid droplets progression in all sarcoidosis patients. The upper sections are mostly dark monophasic, the middle sections are lucent monophasic and the under sections are thought about that unsaturated fatty acids remained for long time and some ones lamellar calcified ones or might be Schaumann Body in the right portion.
Figure 6 and table 3 show abnormal findings of the myelinated nerve fibers surrounding the capillary vessels in the main bronchus. In 36 cases that can be observed amyelinated and myelinated fibers in total 65 sarcoidosis cases, 12 cases (33%) accompanied with swelling of the myelinated fibers (Figure 6e), 4 cases of elongation of one’s (b,d) 2 cases with demyelination and 2 cases with increased amyelinated ones. In 10 cases that can be observed myelinated and amyelinated nerve fibers in total 30 control cases, there are not abnormal findings except for two cases with elongated myelinated ones.
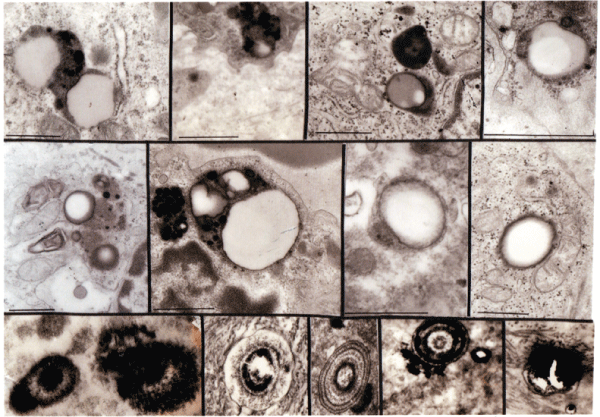
Figure 5: Lipid droplets progression and other corpuscles in all sarcoidosis patients. Under section show a variety of structures and involves lamellar structures.
| Sarcoidosis (65 Cases) | Controls (30 Cases) | |
| Observed myelinated, amyelinated fibers | ||
| Increase of amyelinated fibers | 2 | 0 |
| Swelling of myelinated fibers | 12 (33%) | 0 |
| Elongation of myelinated fibers | 4 | 2 |
| Demyelination | 2 | 0 |
Table 3: Findings of the autonomic nerve fibers of the main bronchus (EM).
Mitochondrion and Figure 7 biphasic lipid substance attached with the defect of mitochondrion’s double membrane. These findings disclose that the degeneration and failure of the double membrane in the mitochondria implicate the lipid droplets formation.
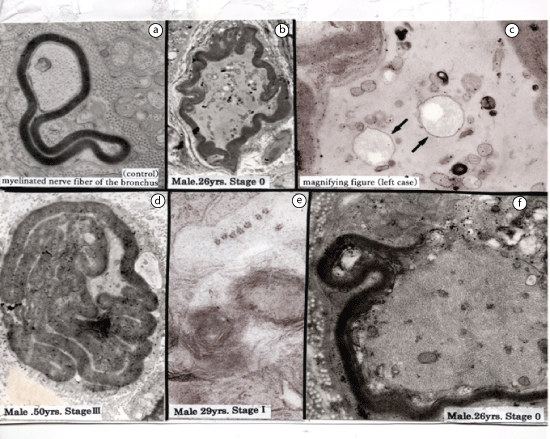
Figure 6: Finding of the autonomic nerve fibers of the main bronchus.
Figure 6a: Normal myelinated nerve fiber.
Figure 6b: Elongation of myelinated fiber.
Fig 6c: Two biphasic lipid droplets in the axon (arrows) (High power view from Figure 6b). Existence of the lipid substances in peripheral nerve fibers is very precious finding.
Fig 6d: Conspicuous elongated myelin fiber.
Fig 6e: Swelling of myelinated fibers.
Fig 6f: Demyelination at the upper portion.
Figure 8 is schema of morphological changes in time process of mitochondria and the lipid droplets. Mitochondria associated with swelling and cristae disappearing make a connection with the lipid droplets accompanied with lysosomes and dark monophasic (arrowhead) and later biphasic crescent figured (arrows).
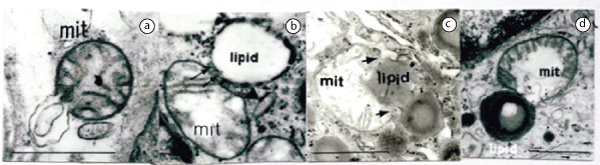
Figure 7: Relationship between mitochondrial membrane and lipid substances in the endothelial cells in early stage of sarcoidosis.
Figure 7a: Appearance of lipid like substance from raptured mitochondrial membrane
Figure 7b: Lipid appearance from the defected double membrane of swollen mitochondrion with disorganized cristae (arrows).
Figure 7c: Swollen mitochondrion with disorganized and disintegrated cristae involves dark monophasic lipid between disappeared mitochondrial membranes (arrows).
Figure 7d: Disorganized cristae containing and swollen mitochondrion involves the biphasic lipid droplet surrounding by lysosome
A lot of degenerated mitochondria are disappeared and finally the lipid droplets are remained as lucid monophasic ones contained neutral fat. Dark monophasic, involving polyunsaturated fatty acid lipid droplets are a crucial role as pathogenesis of sarcoidosis.
We morphologically detected defect of the plasma membrane of the capillary endothelial cells at the broncho-alveolar tract in sarcoidosis patients, and no reports about study of occurrence sites in genesis of sarcoidosis by fine structure. About origin of lipid droplets, we thought that there were 2 kinds of routes from the plasma membrane and mitochondria. Though we need more study about relevance to the plasma membrane and lipid droplets, but we could prove the lipid substances derived from the membranes of the swollen degenerated mitochondria (Figure 7,8).
Phospholipasea A2 (PLA2) are enzymes on target for phospholipids, and catalyzes the hydrolysis membrane glycerophospholipids to liberate free fatty acids and lysophospholopids. This enzyme by gene knockout studies there has been a analysis in diverse kinds of lipid metabolism.
PLA2 expression in sarcoidosis was already reported in 1996 increase of npPLA2 in serum [13], but since then no reports about between sarcoidosis patients and PLA2 . Recently, the patients with membranous nephropathy with sarcoidosis, in which active type showed a high prevalence of PLA2 R have been reported [14]. sPLA2 - IIA is an anti-bacterial sPLA2 that kills invading bacteria by directly degrading bacterial membrane and the serum concentration can say biomarkers for inflammatory and autoimmune diseases [1,15]. sPLA2 - IID is expressed preferentially in dendritic cells in secondary lymphoid organs such as the spleen and lymph nodes suggesting its regulatory role in adaptive immunity [16]. There are several reports about the dendritic cells regulating immune response involving of antigens and its subsequent presentation to T lymphocytes [17-19].
Our proposed lipid droplets [8,9] on growing with the time from dark monophasic the following biphasic, crescent figured lipid droplets are considered as specific polyunsaturated phosphpolipids for sarcoidosis comparing with controls (Table 2).
They may be cardiolipin derivatives with characteristic phase polymorphism, in which cardiolipin take place in the inner mitochondrial membrane and have phase transitions from micellar to lamellar, and from lamellar to hexagonal states are favored by low pH, high ionic strength and high number of acyl groups [20]. The under section in Figure 5, we found lamellar findings that are due to the derivatives of phospholipid cardiolipin. We seemed the pathologically changed phospholipid from mitochondria gradually replace into the high saturated triglycerides.
Mancuso DJ [21] demonstrated that genetic ablation of calcium-independent phospholipase A2 γ induced increase in cardiolipin content and ultrastructurally an appearance of enlarged heteromorphic lamellar mitochondrial structures in hippocampal region of the brain. He emphasized profound changes in mitochondrial lipid metabolism, but not referred to the lipid droplets that I observed in the capillary endothelial cells in sarcoidosis. But the lamellar ones figured at the under section in figure 5 might be mitochondria has not only the major site of cellular ATP production, but also required to sustain neuronal activities and axonal transport of macromolecules and organelles. The functional integrity of mitochondria depends on a dynamic mitochondrial network in cells and interference with these fusion and fission process causes neurodegenerative disorders that are characterized by axonal degeneration of distinct neurons. Our figure (Figure 6f) with demyelination of the autonomic nerves surrounding capillary vessels is precious finding. And present of two biphasic crescent lipid droplets seen in figure 6c is very precious finding for about their origin.
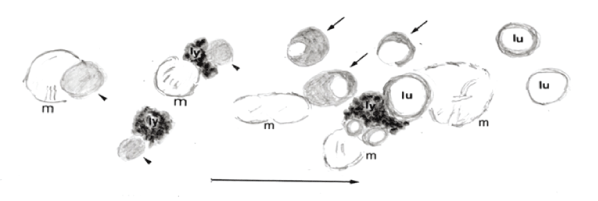
Figure 8: Schema of morphological change in the time process of mitochondria and lipid droplets.
m: mitochondrion arrowhead: unsaturated lipid droplets
ly: lysosome
lu: lucid monophasic lipid droplets ( cf. reference 9. Schema’s improvement)
Recent studies [21-26] have begun to shed light on the function of stomatin-like protein-2 (SLP-2), a widely expressed mitochondrial protein identified in proteome analysis [22]. SLP-2 is involved in the organization of cardiolipin-riched microdomains in mitochondrial membranes and the regulation of mitochondrial biogenesis and function (i.e. energy production, calcium buffering and apoptosis). And SLP-2 may facilitate leading to increased murine T cell signaling and activation by using of confocal microscopy [3].
The impairement in mitochondrial translation at the genetic level correlated with decreased interleukin-2 production in activated T cells, SLP-2 acts as a general regulator of mitochondrial translation. SLP-2 is a mainly mitochondrial protein that forms cardiolipin-riched microdomains [3] and an important player in T cell activation by ensuring sustained T cell receptor signaling and as a potential target for immunomodulation [19,22].
In the symposium on the etiology of sarcoidosis at 36th Japan Society of Sarcoidosis and Other Granulomatous Disorders (Tokyo in 2016) [27], etiological agents except genetic faces were discussed. Propionibacterium acnes, P granulosum, Atopobium spec, Fusobacterium spec. and Mycobacterium were chosen, in which pathogenetic agents have implicated to the obligate anaerobic organisms that able to live only no oxygen surroundings like in the hair rootor with rich fat or oral cavity.
Cardiolipin is a minor component of bacterial and mitochondrial membrane, and a better understanding of cardiolipin is more needful [28], it’ll be very interesting that there will be realized of immune reaction between an anaerobic bacteria’s cardiolipin and mitochondria’s one in the capillary endothelial cells.
In summary, behaviors of PLA2 and SLP-2 by damage of the plasma membrane introduce change of lipid and protein metabolism, and exert an influence upon pathophysiology in sarcoidosis like T cell activation necessary for sarcoidosis granuloma formation.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Mochizuki I, Honda T, Hanaoka M (2018) Pathogenetic Study from Fine Structure of the Capillary Endothelial Cells in the Sarcoidosis Patients-Defects are Observed in the Plasma Membrane, Lipid Droplets and Peripheral Nerve Fibers involving Hilological Consideration for Phospholipase A2 (PLA2) and Stomatin-like Protein 2 (SLP-2). J Infect Pulm Dis 4(1): dx.doi.org/10.16966/2470-3176.135
Copyright: © 2018 Mochizuki I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access