Figure 1: Lactate blood level (mmol/L).


Bernadette Dian Novita1,2* Endang Isbandiati Soediono1 Ni Made Mertaniasih3 Agung Pranoto4 Wuryani5
1Department of Pharmacology and Therapy, Faculty of Medicine Widya Mandala Catholic University Surabaya, Indonesia*Corresponding author: Bernadette Dian Novita, Department of Pharmacology and Therapy, Medical Faculty, Widya Mandala Catholic University Surabaya, Indonesia, Tel: +62-812-3547540; E-mail: diannovitakrisdianto@yahoo.co.id
Metformin (MET) has possibilities to be utilized as an adjunct of TB therapy in controlling the growth of Mycobacterium tuberculosis (Mtb). MET enhances the production of mitochondrial reactive oxygen species and facilitates phagosome-lysosome fusion, those mechanism are important in Mtb elimination. Moreover, MET associated lactic acidosis (MALA) needs to be considered and the incidence of MALA in patients with type 2 DM-TB co-infection remains unknown. This result contributes much to our understanding about the clinical effect of MET use in type 2 DM-TB co-infection.
An observational clinical study was done in type 2 DM newly TB co-infection outpatients at Surabaya Paru Hospital. Patients were divided into two groups. First group was MET group, whom was given MET accompanying insulin and TB treatment regimens, the golden standard therapy of DM-TB co-infection. MET therapy was given for at least 2 months. Second group was non MET group, were given insulin and TB treatment regimens. The lactate levels in both group were measured after 2 months.
From 42 participants, there was no case of lactic acidosis during this study period. Data was normal distribution, thus we continued analysis the difference using paired t-test with 95% confidence. There was no difference in lactate levels (p=0.396) after MET therapy compared to non MET group.3
In this study, there is no evidence that MET therapy is induced lactic acidosis event nor increased of lactate blood level. Thus we concluded that MET use in type 2 DM-TB co-infection not induced lactic acidosis.
Type 2 diabetes mellitus-tuberculosis co-infection; Metformin; Lactic acidosis
Tuberculosis (TB) remains a major source of morbidity and mortality throughout the world; one-third of the world’s population is estimated to be infected with Mycobacterium tuberculosis where by approximately nine million people develop the disease each year, and almost two million die annually as a result [1]. DM-TB (Type 2 diabetes mellitustuberculosis) co-infection is associated with poor glycemic control in DM patients, thus elevated pro inflammatory state [2-4]. People with DM had approximately three times the risk of developing TB disease as people without [2,5-10].
Metformin hydrochloride (MET), biguanide, use in type 2 diabetes mellitus for more than 60 years. MET works by inhibiting the production of hepatic glucose, reducing intestinal glucose absorption, and improving glucose uptake and utilization [11-14]. Recently, by a comprehensive in silico study, MET known has possibilities of utilizing as a combination drug with existing antibiotics for TB therapy [15] and by an extensive in vitro study, MET was reported controlling the growth of drug-resistant M. tuberculosis strains via production of mitochondrial reactive oxygen species and facilitates phagosome-lysosome fusion [16,17].
MET is not metabolised by P450 enzymes [12,13,18], thus it has no interaction with Rifampicin that could decrease the therapy efficacy. However, interaction MET and Rifampicin increases the expression of organic cation transporter (OCT1) and hepatic uptake of metformin, leading to an enhanced glucose-lowering [19,20].
In our previous study, MET were given to type 2 DM newly TB coinfection patients improved the superoxide dismutase (SOD)’s level (unpublished). SOD improvement after MET therapy is predicted to enhance antituberculosis efficacy and considered to reduce the intracellular growth of M. tuberculosis. Collectively, these data indicate that MET is a promising candidate host-adjunctive therapy for enhancing the effective treatment of TB [15,16].
Although MET has several advantages in improving treatment of TB, MET use is still considered to be contraindication in many chronic conditions that may increase the risk of tissue anoxia and the development of MALA, an fatal metabolic condition, especially pulmonary diseases due to potential hypoxia exist [17,21,22].
Lactic acidosis is characterized by an elevated blood lactate concentration ( >45.0 mg/dL or >5.0 mmol/L), decreased blood pH (<7.35), and electrolyte disturbances with anion gap increased [17,22- 29], and has signs and symptoms of inadequate oxygen (hypoxia) such as: shortness of breath; rapid breathing; paleness; sweating; nausea; muscle weakness; abdominal pain; coma [21,25,30].
The objective of this study is to assess the risk of lactic acidosis associated with MET use in patients with type 2 DM newly TB coinfection, combination with golden standard therapies, insulin and TB treatment regimens. Another objective is to evaluate levels of blood lactate, measured at during treatment.
The objective of this study was to identify clinical effect of MET to modulate host immune system and its ability of controlling the growth of intracellular M. tuberculosis. Thus, an observational clinical studies were done and carried out at outpatient ward of Surabaya Paru Hospital and Dr. Ramelan’ Surabaya Naval Hospital. Patient criteria: 1) patient DM with new case of TB co-infection, whom were given insulin and TB treatment regimens; 2) positive M. tuberculosis in sputum smear; 3) patient’s age was 25 to 60 years old; 4) has normal liver function and renal function; 5) not in hypoxia condition, saturation peripheral oxygen ≥92%.
During this clinical study, type 2 DM newly TB co-infection patients were divided into two groups. First group was MET group, whom were given MET accompanying insulin and TB treatment regimens, the golden standard therapy of DM-TB co-infection. MET therapy was given for at least 2 months. Second group, a comparison group, was non-MET group were given insulin and TB treatment regimens.
We evaluated MET combined with insulin and TB treatment regimens. MET therapy was given for at least 2 months. During MET therapy, as a follow up program, patients were weekly physical examination checked, thus signs and symptoms lacto acidosis were monitored. Lactate level was measured as after 2-month MET therapy, for MET group. For comparison, non MET group, whom was given insulin and TB treatment regimens, was also weekly physical examination checked and lactate level was measured after 2-month insulin and anti-TB therapy.
The diagnosis of TB was established by 1) clinical symptoms and signs of TB, such: chronic productive cough, unintentional weight loss; 2) positive sputum smear of acid-fast by microscopic Ziehl-Neelsen-stained sputum slides; and 3) chest radiographs with suggestive features of TB. Diagnosis of DM was established by fasting and 2 hours after meal blood glucose. HbA1c was measured after 2 months MET therapy, as evaluation.
Patients diagnosed with TB were registered and treated with TB treatment regimens for a period of 6 months in accordance to WHO guidelines [31-33]. Management therapy for achieving good glycaemic control was insulin therapy.
This following drugs were used: MET (Metformin(R)), insulin (Humulin(R)), rifampicin (RIF), isoniazid (INH), pyrazinamide (PYR), ethambutol (ETH). MET were given 1000-1500 mg in divided daily dose for at least two months or during intensive phase of TB treatment, accompanying insulin therapy and TB treatment regimens.
After at least 2 months therapy whole blood samples, both groups, were measured by using Biosen C-line glucose and lactate analyzer(R) to test lactate blood levels [25].
During this study period, there were 476 cases of new TB infection and 156 cases (~30%) of that were type 2 DM newly TB co-infection 42 patients, both equally male and female, were eligible participated in this observational studies (Table 1). The condition in both groups were homogenous (p=0.17; p>0.05) using Saphiro Wilk test. The youngest of patient’s age was 26 years old.
| Sex | MET group | Non MET group | ||
| N | Age (ẍ ± SD) | N | Age (ẍ ± SD) | |
| Male | 11 | 44.29 ± 9.76 | 12 | 43.00 ± 9.14 |
| Female | 11 | 43.45 ± 9.10 | 8 | 49.63 ± 6.44 |
| Total | 22 | 43.78 ± 9.08 | 20 | 47.53 ± 7.53 |
Table 1: Characteristic of Patients’ Sex and Ages.
Distribution of patient’s eligible criteria such HbA1c, oxygen saturation, renal function (BUN, creatinine serum) and liver function (SGOT, SGPT) show in Table 2. All data were normal distributed using Saphiro Wilktest, thus, we continued analysis the data for the difference using paired t-test statistics with 95% of confidence.
| Parameters | MET group | Non MET group | p (difference) |
| HbA1c (g/dL) | 8.82 ± 1.91 | 9.52 ± 2.02 | 0.379 |
| Oxygen saturation (SpO2) (%) | 98.06 ± 0.73 | 97.47 ± 0.83 | 0.308 |
| BUN (mg/dL) | 0.95 ± 0.16 | 0.93 ± 0.13 | 0.980 |
| Creatinine serum (U/L) | 23.92 ± 11.92 | 27.3 ± 12.01 | 0.103 |
| SGOT (U/L) | 17.63 ± 6.16 | 14.44 ± 6.48 | 0.354 |
| SGPT (U/L) | 19.22 ± 8.73 | 16.09 ± 7.56 | 0.509 |
Table 2: Distribution of patient’s eligible criteria.
Blood glucose condition for both group were similar (p=0.26), thus we dismissed the influence of hyperglycemia condition to lactate blood level [25,34].
After observation of MET therapy, on divided daily dose of 1000- 1500 mg for at least 2 months accompanying insulin therapy and TB treatment regimens. Lactate blood levels were measured after 2 months MET therapy.
There was no incidence of lactic acidosis event during this period. Additionally, other side effect of MET therapy such gastrointestinal intolerance were also reported. Only two cases of mild gastrointestinal disturbance, such mild frequent diarrhea and nausea/vomiting, were reported.
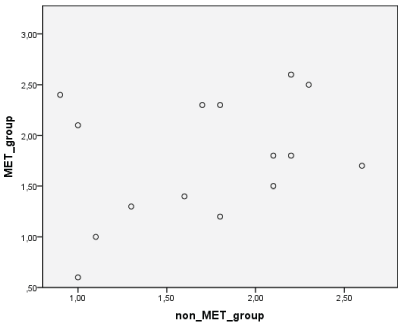
Lactate blood level in both groups were normal distribution, using Saphiro Wilktest (p=0.24; p>0.05), then analyzed the difference between both group using paired t-test (Table 3; Figure 1).

Figure 1: Lactate blood level (mmol/L).
| MET group (mmol/L) | non MET group (mmol/L) | p (difference) |
| 1.77 ± 0.60 | 1.71 ± 0.54 | 0.240 |
| Table 3: Lactate blood level. | ||
Comparing MET group with non-MET group, we concluded there were no statistical significant different of lactate blood level after at least 2 months MET therapy (p>0.05). The level of lactate blood was in normal range (less than 2.50 mmol/L) both in MET group and also non MET group.
The optimum treatment strategy for DM-TB co-infection are not yet known to date. Uncontrollable chronic hyperglycemia in DM patients increases the incidence of TB therapy failure. Besides, DM is also associated with deaths due to TB infection and relapse of TB infection [4,9,35-37]. TB treatment for TB patients with DM has no different from patients without DM [5]. Insulin becomes the main therapy to control hyperglycemia condition. In this study DM-TB patients got insulin and TB treatment, which TB regimen dose referring to World Health Organization (WHO).
Identification of new host-directed therapies that can improve clinical outcomes for DM-TB co-infection patients has been a priority by the World Health Organization, leading to the studies of immunomodulatory agents for adjunct treatment of TB [16,38-40].
MET in some studies, enhances M. tuberculosis-specific host immunity, reduces inflammation and enhances efficacy of TB treatment. MET, which is not metabolized by P450 [12,13,18] enzyme, does not decrease the efficacy of rifampicin. Interaction between MET and rifampicin increases organic cation transporter (OCT)-1 expression which has its role in blocking M. tuberculosis [15,41] transcription. Superoxide Dismutase (SOD) is one essential factor to prevent isoniazid resistency [42] and metformin has been known to increase SOD [43-45], hence it can be concluded that metformin possess the potency to boost OAT effectiveness. MET treatment was associated with improved control of M. tuberculosis infection and decreased disease severity [15,16].
The use of MET may cause side effects such digestion problem (anorexia, nausea, vomit, and diarrhea), lactate level increase, vitamin B12 malabsorption, kidney/heart function problem [12,13]. Though the incidence of Metformin-associated lacto-acidosis (MALA) is low, it must be prevented as it threatens lives. In this study, MALA can occur in very rare situation such as 1) drug-induced hepatitis caused by OAT and/or MET, 2) lung damage which becomes worse causing hypoxia [46,47]. MALA prevention in this study has been determined at the following precondition criteria: 1) minimal to moderate lung lesion; 2) oxygen saturation >92%; 3) normal function of SGOT, and SGPT, and normal kidney function (BUN, SK) (Table 2). Providing consultation, information, and education related to symptoms of lacto-acidosis are also carried out in this study.
Lacto-acidosis is also influenced by high glycemic index. To minimize the bias due to hyperglycemia, HbA1c measurement was done 2 months after the MET therapy accompanying insulin and TB treatment regimens for MET group. HbA1c, for non MET group, was also performed after 2 months of insulin and OAT therapy, or after intensive TB therapy phase was done (Table 2).
There were no MALA cases during this 2-month study, both for MET and non MET groups. It was even proved that blood lactate level was in normal range (<2.50 mmol/L). The blend of MET, insulin and TB treatment was relatively safe for DM-TB patients if some condition was controlled (Table 3).
In this study, we also found that the participants (<5%) in MET group experienced mild gastrointestinal intolerance (nausea and vomit). This can be related to high concentrated MET or glucose metabolism change causing local irritation, fluid retention, and salt mal-absorption, leading to loose stools and diarrhoea [17].
In this study, we were not yet involved TB patients non Type 2 DM, even in our knowledges MET, which has low hypoglycemic effect, could be beneficial in TB patients non DM due to anti-inflammatory effect and increasing efficacy of TB treatment [16,40]. In future, after establishing MET clinical effect in type 2 DM-TB co-infection, we may combine MET therapy with anti TB for TB patients non Type 2 DM in order to evaluate the efficacy of MET therapy in immune modulation.
Lactic acidosis in MET therapy is rare but important adverse event and clearly we need to prevent it. In this case risk study, there is no evidence of MALA. The elevation levels of lactate, compared with placebo were also not occured. Thus we concluded that MET use in type 2 DM-TB coinfection not induced lactic acidosis. Furthermore, this result, due to our limitation in number of participants that involved in the study, need to be confirmed in a cohort study
We thank Sri Hastuti and team (Clinical Pathology, Diagnostic Center Dr. Soetomo Hospital, Surabaya, Indonesia) for her technical assistance. We thank Prof. Dr. Yusak, dr., M.S., Sp.PK (K) for the discussion. This study was supported in part by grant – aid for Ph.D research from Lembaga Pengelola Dana Pendidikan (LPDP), the Ministry of Economic and the Ministry Education, Research and Technology of Republic of Indonesia.
Download Provisional PDF Here
Article Type: Research Article
Citation: Novita BN, Soediono EI, Mertaniasih NM, Pranoto A, Wuryani (2017) Metformin Use in Type 2 Diabetes Mellitus Tuberculosis Co-infection and the Risk of Lactic Acidosis: A Case Risk-Study. J Infect Pulm Dis 3(2): doi http://dx.doi.org/10.16966/2470-3176.124
Copyright: © 2017 Novita BN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access