Abstract
Age related macular degeneration is a common cause of blindness, and its worse manifestation, the choroidal neovascular membrane, can
affect a person’s quality of life, especially in the submacular form. The treatment of the membrane, in the past, was performed only with laser
photocoagulation of the membrane, which reduced the risks of visual loss when treated without delay. If the membrane treated was located in
the macula, central visual area, the outcome was bad despite treatment. Several studies ranging from the Macular Photocoagulation Studies to
intraocular injections nowadays, proved that with prompt treatment with laser photocoagulation, the contrast sensitivity got better within a couple
of years, better that leaving the lesion untreated. Recent studies show how the mechanisms of action of new developed drugs for the treatment
of choroidal neovascular membranes, help improve patient’s outcome.
Ancient treatments, still performed, such as Photodynamic Therapy acted on halting the growth of the membrane, but had to be repeated
several times to achieve the results wanted. Another option that did not last long was Transpupillary Thermotherapy, with the use of nonthermal
laser to treat the choroidal neovascular membrane; treatments such as macular translocation were carried out but had many complications
related to the procedure, and were discontinued; clinical research in pharmacology showed vascular endothelial growth factor as a precursor of
choroidal neovascular lesions.
So the development of pharmacological treatment for the membrane came to the most evolving drugs used in ophthalmology today, starting
with pegaptanib sodium (Macugen) and other drugs under current studies such as bevacizumab (Avastin). Ranibizumab (Lucentis) is also used
for the treatment of the disease, and Aflibercept (Eyelid) was approved and used in many clinical protocols. Corticosteroids were an option for the
treatment of choroidal neovascular membranes, to mention triamcinolone acetate, Ozurdex (dexametasone implant), and Iluvien (fluocinolone
acetone), these last one being delivered as intravitreal implant differently from the others mentioned, delivered as injections. Prompt diagnosis
is desired as many patients arrive past the time of treating the membrane, which may worsen their outcomes. Clinical exam, Oct and Oct
angiography are far more used nowadays. We hope to change that outcome understanding of mechanisms of action of these drugs and trying to
develop new treatments as well as effective medications.
We show a case of subretinal neovascular membrane treated with bevacizumab that failed and developed a recurrent neovascular membrane,
and a new treatment switching the medication was indicated.
Keywords
Age related macular degeneration; Choroidal neovascularization; Vascular endothelial growth factor; Intraocular injections;
Intraocular implants
Abbreviations
ARMD: Age Related Macular Degeneration; CNV: Choroidal Neovascularization; VEGF: Vascular Endothelial Growth Factor;
PDT: Photodynamic Therapy; PEDF: Pigment Epithelium Derived Factor; RPE: Retinal Pigment Epithelium; FDA: Food and Drug Administration;
Oct: Ocular Computerized Tomography; FA: Fluorescein Angiography; OCTA: Oct Angiography; PDGFR: Protein Derived Growth Factor
Receptors; PDGF: Protein Decided Growth Factor
Introduction
Age-related macular degeneration is the most common cause of severe
vision loss in elderly persons in developed countries. Age related macular
degeneration is a painless, irreversible, degenerative eye condition
associated with the damage of photoreceptor cells (Figure1) [1].
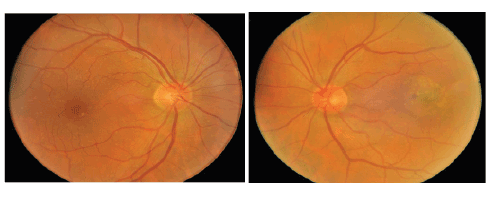
Figure 1: Colour fundus photograph of the right and left eye, respectively, showing normal optic nerves but retinal pigment epithelial changes especially
in the left eye, with was later diagnosed as choroidal neovascular membrane before treatment with infra ocular bevacizumab. Plus, the patient had
metamorphosia in the left eye that motivated him to the consultation with the retina specialist.
Two types of the disease are classified, dry and wet, the first being far
more common, the latter usually worse and associated with metamorphosis
and vision distortion, with loss of central vision. Various agents are used
for treatment, and prevention of the disease, and dietary and life style
considerations may avoid complications of the disease, keeping a stable
visual acuity and quality of life. Early identification of the disease is of
great importance.
The choroidal neovascularization is the primary lesion of age-related
macular degeneration to be treated. The membrane extends anteriorly
through defects in Bruch’s membrane (Figure 2) into the space below the
retinal pigment epithelium and/or neurosensory retina, leading to fluid
accumulation, bleeding or lipids in the subretinal space. Fibrous tissue
may appear, causing central vision loss. The current macular degeneration
related to age treatment in its exudative form is the main challenge in the
world of ophthalmology.
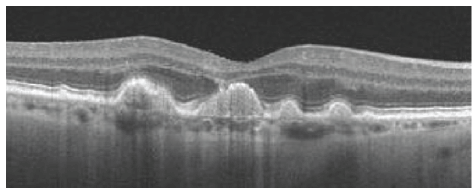
Figure 2: Oct image showing how the choroidal neovascular membrane appears, breaking through the bruchs membrane towards the RPE
Because of recent research into biomaterials and nanotechnology [2]
major advances has been gained in the field of intraocular injections and
delivery systems. New therapies [3,4] are recently presented to the patient
in order to prevent neovascular age-related macular degeneration.
Several mechanisms have been proposed to explain these phenomena.
Vascular endothelial growth factor (VEGF) [5-7] production when blocked
lead to an increase in other angiogenic pathways as a compensatory
mechanism, thus up-regulating VEGF production by macrophages within
choroidal neovascular membranes [8,9].
Photodynamic therapy [10] is a modality is based on the fact that the
choroidal neovascular membranes have tissue characteristics that differ
from normal blood vessels in terms of retaining dye. The treatment,
which uses a combination of drugs and laser therapy, a verteporfin
photosensitive compound that localizes to the target tissue is injected
into a peripheral vein and excited with laser light of a specific wavelength.
Activated verteporfin forms free radicals, and coagulation of the leaking
vessels responsible for cellular injury ensues.
Thermal laser photocoagulation was the treatment of choice for many
years in the management of patients with wet ARMD. In this procedure,
the laser is directed toward the choroidal neovascular membrane, to
destroy it. This procedure, however, has been associated with a high rate
of recurrence [11].
Based on their histology, Gass classified CNVMs into Type 1
and Type 2. Type 1, the subepithelial CNV grows between the
basement membrane of the RPE and the inner collagenous zone of
Bruch’s membrane [12]. The CNVs associated with punctate inner
choroidopathy (PIC), presumed ocular histoplasmosis syndrome
(POHS) and with other PSII are assumed to be Type 2 membranes; so
called inflammatory membranes, and in Type 2, the CNV grows beneath
the sensory retina, lying anteriorly to the RPE.
Other modalities [13-17] of treatment include macular translocation,
submacular surgery and photocoagulation of the feeder vessel, the last
one, guided by green indocyanine, can result in a better outcome, with the
focal treatment of the choroidal neovascular membrane complex.
Auto-antibodies against antiangiogenic agents have been documented
in the systemic circulation of patients undergoing chronic anti-VEGF
therapy for exudative age related macular degeneration, preventing
the action of these agents. Choroidal neovascular lesion composition
might well change with time with more mature and therefore less VEGF
sensitive vessels, so that prompt us to overcome such difficulty with new
agents available [18].
The target layers of their retina and adjacent tissues, represented by the
retinal pigment epithelium (RPE) and the Bruch membrane (BM) [19],
respectively, can be complicated by choroidal neovascular membrane,
formed after damage to the retinal pigment epithelium. A protein, the
pigment epithelium derived factor (PEDF), could have an inhibitory
effect on ocular neovascularization, as well as the VEGF, an angiogenic
factor [20]. The balance between these antiangiogenic and angiogenic
[21] factors may halt or ensue the origin of the choroidal neovascular
membrane. Activation of VEGF induces vascular permeability, endothelial
cell proliferation, and cell migration thus resulting in the formation of a
network of new vessels [22]. Several clinical trials test the relative efficacy
of different drugs and subtypes of the choroidal neovascular membrane [23-25].
Vascular endothelial growth factor (VEGF) has been implicated as a
trigger process in the pathogenesis of ARMD-related choroidal neovascular
membrane. Anti- VEGF agents for the treatment of choroidal neovascular
membrane and are under active clinical investigation, and include antiVEGF
antibodies, gene therapy and protein kinase C inhibition and antiVEGF
aptamer. Many cases are shown to have resulted in a better outcome
after intraocular injections with resolution of the membrane after most of
times some intraocular injections (Figure 3).
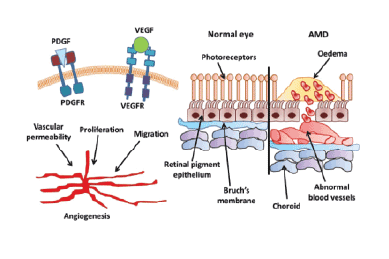
Figure 3: Mechanism of action lf the VEGF and the structures involved.
We show a case of subretinal neovascular membrane treated with
bevacizumab that fails with recurrent neovascular membrane, and a new
treatment switching the medication was indicated.
Case Report
We performed bevacizumab [2] intraocular injections in a patient with
metamorphosia and CNV diagnosed on December, 2015. The patient was
free of systemic symptoms, and had solely eye symptoms, manifested by
metamorphosis in the left eye. The patient had visual acuity of counting
fingers at 15 centimeters in the left eye, and 20/40 in the right eye. He
had three injections of bevacizumab in December, 2015 followed by two
more injections in January and February, 2016. He used to see a central
blur out of the left eye and the right eye was asymptomatic. The patient
was submitted to Oct - ocular computerized tomography and fluorescein
angiography, before and after treatment. The patient was still counting
fingers, but without the blur, and felt that his visual acuity got a lot
better. The Oct pre-treatment showed a foveal minimum thickness of 313
microns and after the injections that increased to 340 microns, despite
he noticed better visual acuity. The fluorescein angiography showed
recurrent leakage from the membrane. Because of the risk of worsening
both clinically and anatomically, and mainly because the lesion was still
leaking and active, we decided to switch the drug to Ranibizumab, with
the aim of halting the CNV formation quicker, to avoid worsening of the
patient´s visual acuity (Figure 4).
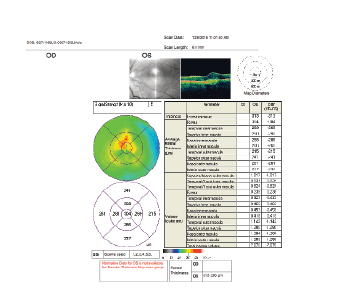
Figure 4: Oct of the left eye showing, among other measures, a foveal thickness of 313 microns, before three bevacizumab treatments.
Discussion
Antiangiogenic Compounds mostly used [26-28]:
Pegaptanib sodium
Pegaptanib [29] sodium is an aptamer against VEGF165, the isoform
identified with pathological angiogenesis, the aptamer being an
oligonucleotide that acts like a high affinity antibody to VEGF, neutralizing
it before it can contact its receptor.
Ranibizumab
Ranibizumab is a recombinant monoclonal antibody Fab fragment
that neutralizes all active forms of VEGF-The FDA approved the use of
ranibizumab for the treatment of all angiographic subtypes of subfoveal
neovascular ARMD.
Bevacizumab [2]
Bevacizumab is a humanized, recombinant monoclonal
immunoglobulin G (IgG) antibody that binds and inhibits all VEGF
isoforms and is currently approved for systemic use in metastatic
colorectal cancer and non–small cell lung cancer, and is used for CNV
secondary to ARMD since 2005. Most of the reports of bevacizumab are
uncontrolled, open-label case series that have suggested functional and
anatomical efficacy, short-term safety, and lower costs (Figures 5-9).
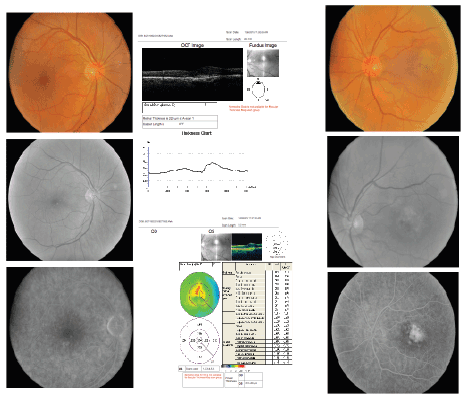
Figure 5: Right and Left eye, respectively, showing color fundus photographs (above), followed by monochromatic (middle) and
late FAs (below), before treatment with bevacizumab for CNV in the left eye.
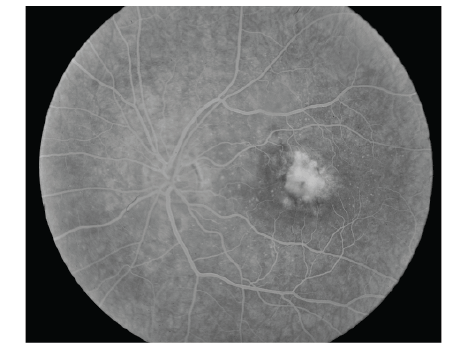
Figure 6: Left late angiographic photograph of the left eye before treatment with bevacizumab intraocular injection. The leak involves the foveal area
and spreads irregularly, making the diagnosis of an occult CNV.
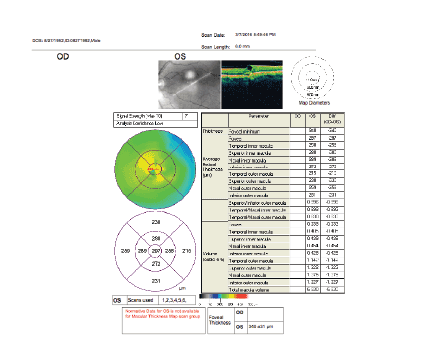
Figure 7: Oct of the left eye showing, among other measures, a foveal thickness of 313 microns, after three bevacizumab treatments
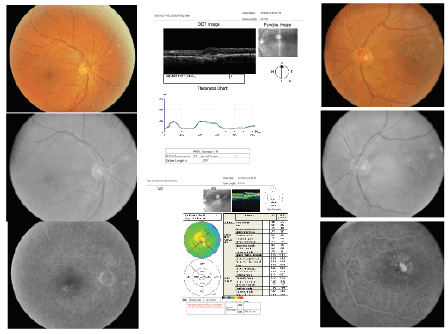
Figure 8: Right and Left eye, respectively, showing color fundus photographs (above), followed by monochromatic (middle) and
late FAs (below), after treatment with bevacizumab for CNV in the left eye.
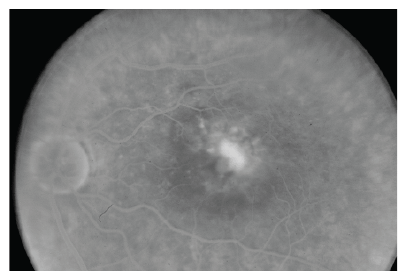
Figure 9: Left late angiographic photograph of the left eye after treatment with bevacizumab intraocular injection. The leak involves the foveal area
and spreads irregularly, making the diagnosis of an occult CNV. Note that the membrane did not shrink enough and the patient was indicated to
switching therapy with another antiangiogenic drug, namely ranibizumab.
Aflibercept
The VIEW 1 and VIEW 2, two similarly designed double- masked,
randomized multicenter clinical trials, demonstrated that intravitreal
aflibercept dosed monthly or every 2 months after a loading dose of 3
monthly doses was non inferior to monthly ranibizumab.
The major concern for ocular complications following intravitreal
anti-VEGF injections was for endophthalmitis, and non-infectious
inflammation to the biologic anti-VEGF agents; other complications
could be retinal tears, retinal detachment, tears of the retinal pigment
epithelium; elevated intraocular pressure and cataracts.
Important designed studies
The Comparison of Age-Related Macular Degeneration Treatment
Trials (CATT) research group demonstrated that at 1 year, bevacizumab
and ranibizumab had equivalent effects on visual acuity when administered
according to the same schedule. Bevacizumab administered monthly was
equivalent to ranibizumab administered monthly, with 8.0 and 8.5 letters
gained, respectively.
To date, there are several studies and many more coming on the way,
demonstrating the efficacy of intravitreal injections [30]:
The MARINA (Minimally classic/occult trial of the Anti- VEGF
antibody Ranibizumab In the treatment of Neovascular Age-related
Macular Degeneration) study demonstrated the intravitreal administration
of ranibizumab for two years to prevent vision loss while improving the
mean visual acuity with low rates of serious adverse events, in patients
with minimally classic or occult choroidal neovascularization secondary
to age-related macular degeneration.
The ANCHOR ( anti-VEGF antibody for the treatment of predominantly
classic choroidal neovascularization in age-related macular degeneration)
study demonstrated that ranibizumab provided greater clinical benefit
after two years than verteporfin PDT in patients with age related macular
degeneration with new-onset, predominantly classic CNV.
The PRONTO (Prospective Optical coherence tomography imaging of
patients with Neovascular age- related macular degeneration Treatment
with intraocular ranibizumab) study used an Optical Coherence
Tomography (OCT)-guided variable dosing regimen with intravitreal
ranibizumab. During the first year, retreatment with ranibizumab
was performed at each monthly visit if any criterion was fulfilled such
as an increase in OCT-CFT of at least 100 μm or a loss of 5 letters or
more. During the second year, the retreatment criteria were amended
to include retreatment if any qualitative increase in the amount of fluid
was detected using OCT. This study demonstrated that at month 24, the
mean VA improved by 11.1 letters and the CFT decreased by 212 μm.
The VA improved by 15 letters or more in 43% of patients. These VA and
OCT outcomes were achieved with an average of 9.9 injections over 24
months. As-needed (PRN), OCT-guided variable dosing with intravitreal
ranibizumab resulted in VA outcomes comparable to the outcomes from
the phase III clinical studies (monthly injection), but fewer intravitreal
injections were required.
Pegaptanib appears to be an effective therapy for AMD. However, it
does not lead to any improvement in the mean visual acuity.
The PIER (Phase IIIb, multicenter, randomized, double- masked, sham
Injection-controlled study of the Efficacy and safety of Ranibizumab in
subjects with subfoveal CNV with or without classic CNV secondary to
age- related macular degeneration) study demonstrated that ranibizumab
administered monthly for three months and then quarterly provided
visual acuity benefits to patients with neovascular age related macular
degeneration and was well tolerated. However, the observations from the
MARINA and ANCHOR trials suggest that the PIER regimen of dosing
every three months after three monthly doses provides less benefit in
terms of visual acuity on average than continued monthly dosing. Monthly
dosing may be necessary in some patients to achieve maximal treatment
benefit from ranibizumab.
The CLEAR-IT (Clinical Evaluation of Anti-angiogenesis in the Retina
Intravitreal Trial) study demonstrated that PRN dosing of VEGF Trap-Eye
after 12 weeks of monthly or quarterly fixed dosing maintained clinically
and statistically significant improvements in vision and retinal thickness
until at least week 52 in patients with neovascular AMD, with a low
frequency of reinjection.
VEGF Trap-Eye was generally well tolerated, with a safety profile similar
to that reported with other intravitreally administered anti-VEGF agents.
The LEVEL (Evaluation of Efficacy and Safety in Maintaining Visual
Acuity with Sequential Treatment of Neovascular age related macular
degeneration) study assessed the efficacy of pegaptanib as maintenance
therapy in AMD patients who experienced a clinical improvement in
disease following an induction phase. The induction maintenance using
nonselective, followed by selective VEGF inhibitors should be considered
for the treatment of AMD. Such an approach has special relevance for
patients with cardiovascular co morbidities who require anti-VEGF drugs
to manage their AMD.
The VISION (VEGF Inhibition Study In Ocular Neovascularization)
study demonstrated that in the group given pegaptanib at 0.3 mg, 70% of
patients lost fewer than 15 letters of visual acuity, as compared with 55%
among the controls (P < 0.001).
Conclusion
The knowledge of the molecular physiopathology of the CNV [31]
prompted the treatment of different subtypes of its neovascular form.
Several studies, some mentioned above, contributed for backing up the
use of these agents worldwide, even though some studies are still on the
way and others are to come. But the future promises better results, and the
combination of treatments with other drugs and also old treatments such
as laser and PDT and others are still applied.
Pharmacology gives us a broad spectrum of options, and together
with other related specialties, to date nanotechnology [25], gives us a
better appreciation of the future. Some patients are prone to better results
rather than other patients, and that may be corrected with different drugs
switched after a good clinical evaluation, added with Octs and FA, as well
as other diagnosing tools, such as Oct angiography.
We need more studies to compare, especially with the new OctA tool,
and that will help us in the follow up of these patients and management of
new patients in order to avoid the growth of the CNV.
Despite recurrent rates could be high and switching medications may
be necessary for the sake of the patient’s vision, that decision should
be taken without delay, to avoid growth of the choroidal neovascular
membrane ant its recurrence with devastating effects specially if located
on the foveal area. We showed a case that needed retreatment with a new
drug, switched to another medication able to perform better.










