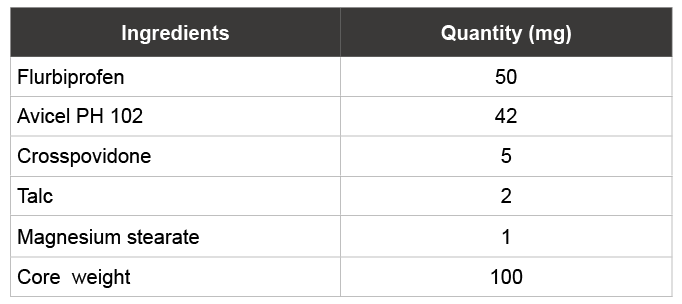
Table 1: Composition and characterization of FLB core tablets

Sateesh Kumar Vemula1,2* Vijaya Kumar Bontha3
1Department of Pharmacy, College of Medical and Health Sciences, Wollega University, Post Box No: 395, Nekemte, Ethiopia*Corresponding author: Sateesh Kumar Vemula, Department of Pharmacy, College of Medical and Health Sciences, Wollega University, Post Box No: 395, Nekemte, Ethiopia, E-mail: vemulasatish15@gmail.com
Pulsatile drug delivery using non-steroidal anti-inflammatory drugs to exploit the treatment of colonic inflammation and rheumatoid arthritis is one of the ongoing pharmaceutical researches in the modified release systems. The intention of present study is to develop the flurbiprofen pulsatile tablets using double-compression coating method to give the colon-specific chronopharmaceutical drug release. In this study, flurbiprofen core tablets were prepared by direct compression method and then compression coated with two layers. Core tablets were primarily coated with inner-compression coat made of sodium starch glycolate as swelling layer and then with outer-compression coat (release-controlling layer) using hydroxypropyl methylcellulose K 15 M or sodium alginate. Then the tablets were evaluated for various physical and in vitro evaluations. From the in vitro drug release studies, F5 tablets showed 4.74 ± 0.21% drug release in 5 h and it was improved to 99.02 ± 0.76% in 24 h. The mean dissolution time of all formulations was found to be 4.50-14.72 h and it was higher for formulations with HPMC K 15 M compared to sodium alginate. Time in hours to take 10% and 80% drug release explained the ability of prolonged release and they were found to be 5.7 h and 21.2 h respectively for best formulation F5. From the stability studies, similarity factor (f2 ) was found as 80.56, which is more than 50 indicates similarity between the dissolution profile before and after storage. Hence the development of flurbiprofen double-compression coated pulsatile tablets is a promising way to control the drug release as per therapeutic requirement.
Core tablets; Direct compression; Double-compression coating; Mean dissolution time; Release controlling layer; Similarity factor; Swelling layer
Development of pulsatile drug delivery system is one of the promising time- and site-specific systems, required for those diseases that sensitive to circadian rhythms and colonic diseases [1]. Pulsatile colon-specific drug delivery has significant role in the treatment of diseases like bronchial asthma, hypertension, angina pectoris, allergic rhinitis and rheumatoid arthritis with mainly night or early morning symptoms [2]. Inclusion of required lag time into the tablets is highly depending on the nature of therapeutic application and is depending upon the polymer used and thickness of compression coating [3]. Among the recent research on colon-specific pulsatile drug delivery systems, some of the examples are colon-specific double-compression coated pulsatile tablets of ketorolac tromethamine [4], colon targeted pulsatile system of flurbiprofen for colonic inflammation [5], pulsatile systems for targeted drug delivery of celecoxib for prophylaxis of colorectal cancer [6], time and pH dependent colon specific, pulsatile delivery of theophylline for nocturnal asthma [7].
Compression coating method i.e., solvent less coating is a safe, stable and inexpensive method when compared to solvent film coating that doesn’t require special coating equipment [8]. Recent research examples reported on compression coated tablets are flurbiprofen-sodium alginate compression coated tablets [3], ketorolac tromethamine-hydroxypropyl methylcellulose compression coated tablets [8], ketorolac tromethaminesodium alginate compression coated tablets [9], flurbiprofen-guar gum compression coated tablets [10], 5-fluorouracil-hydroxypropyl methylcellulose compression coated tablets [11], ketorolac tromethamineguar gum compression coated tablets [12], flurbiprofen doublecompression coated mini-tablets [13] etc. Even though sufficient research was done on compression coated tablets, minute information is existing on double compression coating technology. Hence it is designed to formulate the double-compression coated pulsatile colon-specific tablets using flurbiprofen (FLB) as a model drug. FLB is a non-steroidal antiinflammatory drug and is one of the widely used drugs for treatment of rheumatoid arthritis and to treat colonic inflammation [14,15].
Flurbiprofen was a gift sample from FDC Limited, Mumbai, India. Hydroxypropyl methylcellulose K 15 M (HPMC K 15 M) and Sodium alginate (SA) are obtained as a gift sample from MSN Laboratories, Hyderabad, India. All other chemicals used were of analytical grade.
FLB core tablets were prepared using direct compression method prepare the in which 50 mg of FLB was used and the final core weight was adjusted to 100 mg (Table 1). Accurately weighed amount of FLB and excipients were passed through 60 # sieve, blended for 5-10 min in poly bag, then lubricated the powder and finally compressed into tablets with 6 mm round flat punches on 8-station rotary tablet compression machine, Riddhi Pvt Ltd, India.
To achieve the pulsatile colon-specific drug release, core tablets were subjected to compression coating by double compression coating method (different compositions were given in Table 2). Core tablets were compression coated with two consecutive layers; sodium starch glycolate as an inner swelling layer (100 mg weight) and SA/HPMC K 15 M as an outer release controlling coating (100 mg weight). Cores were compression coated using 8 mm (inner compression coat) and10 mm (outer compression coat) circular flat punches by placing half of the coating material in die cavity, then cautious placing of cores in middle and finally placing the remaining half of coating material.
The prepared tablets were studied for their physical properties like weight variation, hardness, friability and drug content uniformity. For estimating weight variation, 20 tablets of each formulation were weighed using an Electronic weighing balance (AW 120, Shimadzu Corporation, Japan). The strength of tablet is expressed by measuring hardness and friability. The hardness of ten tablets was measured using Monsanto tablet hardness tester. Friability was determined using six tablets in a Roche friabilator (Electro lab, Mumbai, India) that operated for 4 min at 25 rpm.
Randomly selected ten tablets were crushed and the powder equivalent to 100 mg of drug was accurately weighed and transferred to a 100 ml volumetric flask. Initially about 50 ml of mobile phase was added to the volumetric flask and allowed to stand for 6-8 h with intermittent shaking to ensure complete solubility of the drug. Then the volume was made up to 100 ml with mobile phase followed by filtration and analysis for FLB content by the previously developed HPLC method [5]. Mobile phase used for the analysis consists of phosphate buffer: acetonitrile aqueous solution in the ratio of 35:65. They were filtered before use through a 0.45 µm membrane filter and pumped through the column Symmetry C18 (X Terra, 4.6 × 150 mm) 5 µm, at a flow rate of 1 ml/min. The analysis was performed at ambient temperature and the run time was set to 8 min. The eluents were monitored at 254 nm using UV detector.

Table 1: Composition and characterization of FLB core tablets
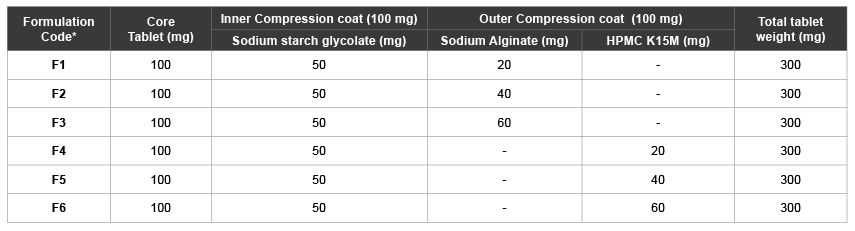
Table 2: Composition of FLB double-compression coated tablets
*Each compression coat formulation contains 1% Magnesium stearate, 2% Talc and Avicel PH 102 to make up the compression coat weight.
USP type II dissolution apparatus (Electro lab, TDT-08 L) was used to conduct the in vitro drug release studies of prepared double compression coated tablets (n=6) at 50 rpm speed and 37°C ± 0.5°C temperature in 900 ml dissolution media (0.1 N HCl for first 2 h; 3-4 h in 5.5 pH buffer and then in phosphate buffer pH 7.4 from 5 to 24 h). At specific time intervals 5 ml sample was withdrawn and replaced with the same volume of fresh pre-warmed dissolution medium, filtered and analyzed the HPLC method at 254 nm using UV detector.
Data obtained from dissolution studies was used to calculate the mean dissolution time (MDT) i.e., the sum of different release fraction periods during dissolution studies divided by the initial loading dose [16], T10% and T80% (time in hours to take 10% and 80% drug release, respectively) to explain the drug release from compression-coated tablets [17].
Using ICH guidelines, the stability studies were planned to assess the stability of FLB in compression coated tablets. Three replicates of F5 tablets were sealed in aluminum coated polyethylene pack and stored at 40 ± 2°C and 75 ± 5% RH in the humidity chamber for six months [18]. Samples were collected after six months of storage and evaluated for the drug content and in vitro dissolution rate [19]. Then the data was statistically analyzed using paired t-test to test the significance of difference at level of significance 0.05. To prove the stability of dosage form, the similarity factor (f2 ) was calculated between dissolution rates of F5 tablets before and after storage [20].
The physical parameters were determined for prepared double compression coated tablets and showed in Table 3. The pharmacopoeial limits for the tablet weight variation test were not more than 10% of the average weight and found to be 299.42 ± 2.75 - 300.78 ± 2.07 mg. The tablet hardness and friability were found to be around 6 kg/cm2 and below 0.5%, demonstrating the integrity and strength of tablets. The assay of prepared tablets was found to contain 98.78 ± 1.08%-99.94 ± 1.18%.
To evaluate the effect of double compression coating on the FLB core tablets, in vitro drug release studies of the F1-F6 formulations were conducted in various pH dissolution media and the results were shown in Figure 1. Among all, F5 formulation showed 4.74 ± 0.21% drug release in 5 h and it was continuously expanded to 99.02 ± 0.76% in 24 h. In these, SA formulations showed some compressibility problem and also less effective as sustained release polymer where as formulations containing HPMC K 15 M showed good control in drug release with desirable compressibility. The effect of double compression coating on the drug release was given in Figure 2 when compared to core tablets.
The MDT values of all formulations were found to be 4.50-14.72 h. The T10% and T80% values of best formulation F5 was found to be 5.7 h and 21.2 h respectively (Figure 3). These values were found to be greater for HPMC K 15 M when compared to SA. This indicates the good release retardation power of HPMC K 15 M.
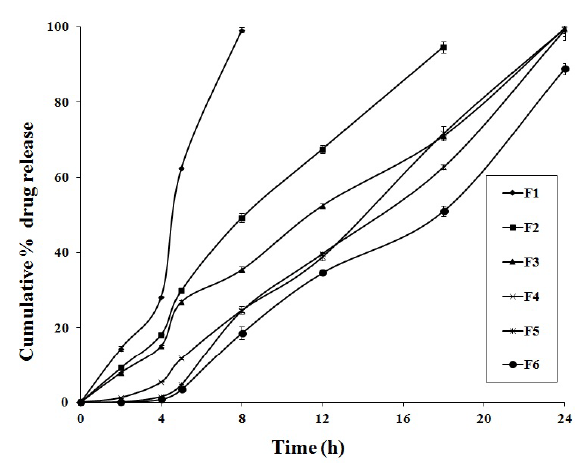
Figure 1: Drug Release profile from FLB colon-specific doublecompression coated tablets (F1-F6).
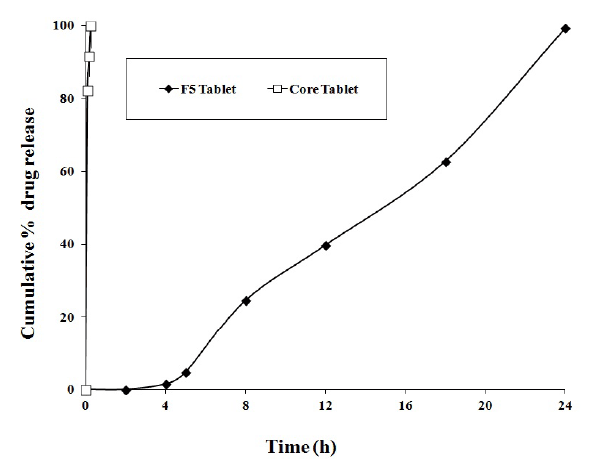
Figure 2: Comparison of drug release from core and F5 colon-specific double-compression coated FLB tablets.
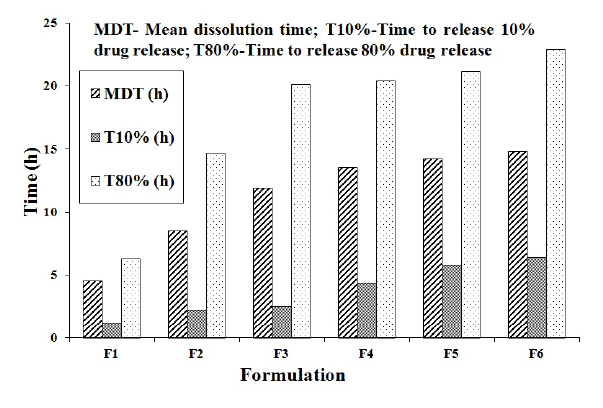
Figure 3: Drug Release parameters of FLB colon-specific doublecompression coated tablets (F1-F6).
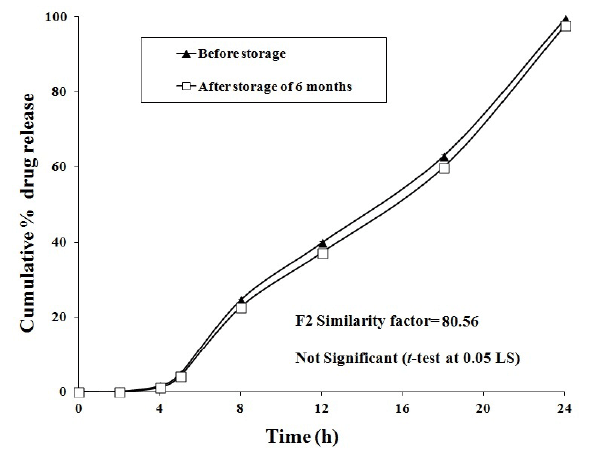
Figure 4: Stability studies of F5 colon-specific double-compression coated FLB tablets.
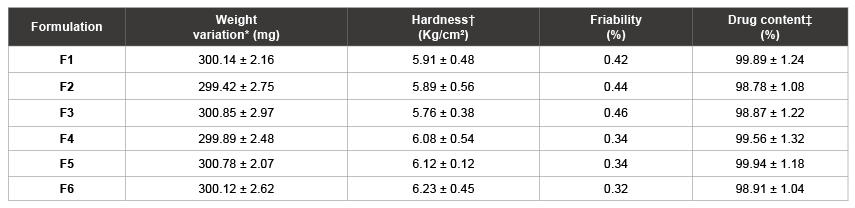
Table 3: Physical properties of FLB compression coated tablets
*
All values represent mean ± standard deviation, n=20;
†All values represent mean ± standard deviation, n=6;
‡ All values represent mean ± standard deviation, n=3
Stability studies of F5 formulation was carried out at 40 ± 2°C and 75 ± 5% RH for six months to assess drug stability. After storage of six months, the formulation was subjected to a drug assay and in vitro dissolution studies and the results were shown in Figure 4. From the statistical analysis there was no significant difference between before and after storage (P<0.05). The similarity factor (f2 ) between dissolution profiles of F5 formulation before and after storage was found to be 80.56.
An effort was made to formulate flurbiprofen double compression coated tablets to gain the pulsatile drug release to improve the therapeutic drug levels in colon by minimizing the drug release in stomach and small intestine using inner compression coat of swelling layer and outer compression coat that act as release controlling layer. In the present study, FLB double compression coated tablets were prepared and evaluated for various parameters. One of the major challenges to the formulation scientists is to formulate a tablet with acceptable physical properties and mechanical strength that could not adversely affect the drug release pattern. To check the compliance with pharmacopoeial standards, physical properties like weight variation, thickness, hardness and friability were determined. In weight variation test, the average percentage deviation of all tablet formulations was found to be within the pharmacopoeial limits. All tablet formulations were found uniform in hardness, friability and drug content.
Among the prepared FLB double compression coated tablets, F1-F3 formulations were explain the effect of SA while F4-F6 shown the effect of HPMC K 15 M on drug release. From the in vitro drug release studies, tablets with HPMC K 15 M as outer compression coat was superior not only in the drug release control but also possess the acceptable compressibility and mechanical integrity. But SA formulations were failed in to hold the sufficient compressibility and they required more SA concentration in outer layer to control the drug release as HPMC K 15 M. By comparing the dissolution results, F5 tablets containing 40 mg of HPMC K 15 M was judged as the best formulation that not only retard the drug release in first 5 h but also showed the progressive drug release in colon. Similar type of results were observed in a study developed by Veerareddy PR et al. [5] for both flurbiprofen and Vemula SK [8] for ketorolac tromethamine using HPMC K 4 M as the compression coating polymer, but in the present study it requires low polymer concentration due to high viscosity grade of HPMC.
The drug release kinetics studies revealed high correlation coefficient values for zero order than first order indicating that the drug release from matrix tablets followed zero order profile. Zero order release was also observed in a study with 5-fluorouracil using HPMC in the compression coat [11]. The high regression value of Higuchi model ensured that the release of drug from matrix tablets followed diffusion mechanism. The n values calculated for different formulations indicate a supercase-II transport. The MDT was higher for formulations with HPMC K 15 M compared to SA that indicating better controlled release. T10% and T80% explained the ability of prolonged release and they were high for HPMC K 15 M when compared to SA. From the stability studies, the data showed that there was no significant change in formulation in the sense of drug content and dissolution behavior. The similarity factor (f2 ) was found as 80.56 that are more than 50 indicate similarity between the dissolution profile before and after storage.
From the above results, it was concluded that the double-compression coated tablets were suitable to gain the pulsatile colon-specific drug release with negligible drug release in stomach and small intestine. They were preferable not only to treat colonic inflammation but also to treat rheumatoid arthritis with mainly night or early morning symptoms due to incorporated 5 h lag time.
The present study is aimed to formulate the FLB pulsatile doublecompression coated tablets using sodium starch glycolate as inner compression coat and SA/HPMC K 15 M as the outer compression coat. From the physical characterization and in vitro drug release studies, F5 formulation was considered as the best formulation to gain colonspecific drug release with acceptable mechanical strength. In conclusion, development of the double-compression coated tablets is a suitable approach to gain the pulsatile colon-specific FLB release to maximize the therapy for colonic inflammation and rheumatoid arthritis that sensitive to circadian rhythms.
The authors acknowledge the FDC Limited and MSN Laboratories India for gift samples. The authors also thank to Management Chaitanya College of Pharmacy Education and Research for providing compression facilities.
The authors report no conflicts of interest.
Download Provisional PDF Here
Article Type: Research Article
Citation: Vemula SK, Bontha VK (2016) Flurbiprofen Pulsatile Colon-Specific Delivery: Formulation and Evaluation of Double-Compression Coated Tablets. J Pharm Anal Insights 1(1): doi http://dx.doi. org/10.16966/2471-8122.104
Copyright: © 2016 Vemula SK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access