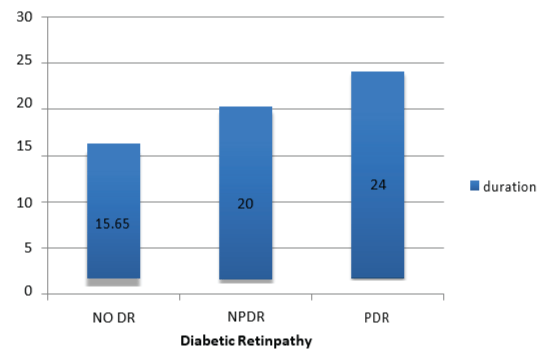
Figure 1: Association between diabetic retinopathy (DR) and diabetes mellitus (DM) duration.


Elagamy A1*,# Al Enazy BR2# AL Zaaidi SE3
1Amira Elagamy, Assistant Professor of Ophthalmology, Department of Optometry and Vision Sciences, College of Applied Medical Sciences, King Saud University, Saudi Arabia and Mansoura Ophthalmic Center, Faculty of Medicine, Mansoura University, Egypt*Corresponding author: Amira Elagamy, Assistant Professor of Ophthalmology, Department of Optometry and Vision Sciences, College of Applied Medical Sciences, King Saud University, Saudi Arabia and Mansoura Ophthalmic Center, Faculty of Medicine, Mansoura University, Egypt, E-mail: aelagamy@ksu.edu.sa
Purpose: This study was conducted to assess the association between dyslipidaemia, blood pressure and diabetic retinopathy (DR) in type 2 diabetic patients. In addition, evaluation of the association between dyslipidemia and diabetic macular edema (DME) was performed.
Design: This study was a retrospective and cross-sectional design, observational, and quantitative study.
Subjects and Methods: This study included 800 patients with type 2 DM (386 males and 414 females). The age range was between 35-55 years. They were divided into two groups: 400 patients have DR (group 1), and 400 patients have not been previously diagnosed with DR (group 2). History and medical records of each diabetic patient were reviewed. Also, ophthalmological records of all subjects were reviewed including visual acuity and Intra Ocular Pressure (IOP) measurement using Air puff tonometer, macular thickness measured by Fourier-Domain Optical Coherence Tomography (OCT) and retinal imaging performed using Optomap 200° Ultra-Widefield (UWF) Digital retinal scan. Measurements of blood pressure for all patients were reviewed. The last blood samples were reviewed for all subjects between 6 months to 1 year prior to our ophthalmic examination. Serum total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C), low lipoprotein density-cholesterol (LDL-C), triglycerides (TG), plasma glucose and glycated hemoglobin (HbA1c) were taken. In addition, body mass index (BMI) and 10-year risk of Atherosclerotic Cardiovascular Disease (ASCVD) were calculated.
Results: There was a statistically significant relationship between DM duration and DR (P<0.0001). Group 1 showed a statistically significant higher systolic and diastolic blood pressure than group 2 (P<0.0001) & (P=0.0289) respectively. Also, group 1 documented a statistically significant higher levels of TC, LDL-C and low levels of HDL-C (0.0013*, < 0.0001* and 0.0027*) respectively. But no statistically significant difference was detected between the 2 groups relating to TG (P=0.5478). Besides, patients with DME had a statistically significant high LDL-C and low HDL-C levels than patients without DME (P<0.002) and (P<0.003) respectively.
Conclusion: This study documented significant association between DR and dyslipidemia in type 2 diabetic patients. In addition, the study confirmed that patients with DME had significant high LDL-C level and low HDL-C level than patients without DME. Moreover, the current study demonstrated significant association between DR and hypertension. Therefore, efficient control of hyperglycemia, dyslipidemia and hypertension would be of great value in delaying the progression of DR in these patients.
Diabetic retinopathy; Diabetic macular edema; Dyslipidemia; Hypertension
Diabetes Mellitus (DM); High Serum Low-Density-Lipoprotein Cholesterol (LDL-C); Low Serum High-Density-Lipoprotein Cholesterol (HDL-C); Diabetic Retinopathy (DR); American Diabetes Association (ADA); Total Cholesterol (TC); Triglyceride (TG); Diabetic Macular Edema (DME); Intra Ocular Pressure (IOP); Ultra-Widefield (UWF); Optical Coherence Tomography (OCT); National Screening Committee (NSC); Glycated Haemoglobin (HbA1c); Non-Proliferative Diabetic Retinopathy (NPDR); Proliferative Diabetic Retinopathy (PDR); Atherosclerotic Cardiovascular Disease (ASCVD); Body Mass Index (BMI); Blood Pressure (BP); Statistical Package for Social Sciences (SPSS); United Kingdom Prospective Diabetes Study (UKPDS); Multi-Ethnic Study of Atherosclerosis (MESA); Cardiovascular Health Study (CHS); Early Treatment Diabetic Retinopathy Study (ETDRS); Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR)
Diabetes Mellitus (DM) is a severe metabolic illness, with an increasing frequency worldwide [1]. In Kingdom of Saudi Arabia, the incidence of DM was evaluated as 23.7% with more significant predominance in males compared to females (26.2% and 21.5% respectively) [2].
Metabolic syndrome is identified as an association of type 2 DM and other conditions such as hypertension, high serum low-densitylipoprotein cholesterol (LDL-C) concentration, and low serum highdensity-lipoprotein cholesterol (HDL-C) concentration [3].
Several studies [4,5] have demonstrated that chronic hyperglycemia, hypertension and hyperlipidemia contribute to the pathogenesis of Diabetic retinopathy (DR) which is one of the most common causes of visual impairment in people of working age, affecting both genders equally. The onset and deterioration of DR can be delayed by effective metabolic control. Elevated glucose levels cause retinal microangiopathy but the strict pathogenesis by which hyperglycemia leads to vascular damage seen in DR is ill defined. On the other hand, many biochemical pathways have been proposed to prove association between hyperglycemia and retinal microvascular abnormalities [6].
As specified by the American Diabetes Association (ADA) 2016 [7] hypercholesterolemia was demarcated as total cholesterol (TC) >200 mg/dl, high LDL-C when value >100 mg/dl, hypertriglyceridemia as triglycerides (TG) >150 mg/dl and low HDL-C when value <40 mg/ dl. Dyslipidemia was well-defined by the presence of one or more than one abnormal serum lipid concentrations. Prescription of Statin treatment should be of great value in these cases.
The aim of this study was to assess the association between dyslipidemia, blood pressure and DR in type 2 diabetic patients. In addition, evaluation of the association between dyslipidemia and diabetic macular edema (DME) was performed.
This study was a retrospective and cross-sectional design, observational, and quantitative study.
This study got the approval of Research Ethics Committee of College of Applied Medical Sciences, King Saud University and Research Ethics Committee in Prince Sultan Military Medical City, Riyadh, Saudi Arabia. All procedures in this study were performed in accordance with Helsinki declaration and its later amendments. All the participants signed comprehensive consent after explanation of the possible consequences of the study prior to investigations. The participants were consecutively enrolled from the Department of Ophthalmology, Diabetic Center, Prince Sultan Military Medical City, Riyadh, Saudi Arabia from January 2015 to April 2015. Inclusion criteria included patients with type 2 DM aged 35 years. A total of 800 patients (386 males and 414 females) were enrolled in this study. The age range was between 35-55 years.
They were divided into two groups:
Group 1: 400 patients with type 2 DM have been labelled as DR by an ophthalmologist (Retina Specialist).
Group 2: 400 patients with type 2 DM had not been previously diagnosed with retinopathy, nephropathy or other diabetes complications.
Exclusion criteria included patients with retinal disorders like vein/ artery occlusion, retinitis pigmentosa, vitreo-retinal degeneration and dystrophies. In addition, all secondary causes of retinal neovascularization, history of uveitis, history of glaucoma, type 1 DM patients, and patients <35 years old were excluded.
For each patient, history and medical records were reviewed to determine the DM duration. Also, ophthalmological records of all subjects were reviewed including visual acuity assessment using Snellen visual acuity chart, refraction using Auto-refractometer (Topcon) and Intra Ocular Pressure (IOP) measurement using Air puff tonometer, macular thickness measured by Fourier-Domain Optical Coherence Tomography (OCT) (RTVue-100 software version: 6.8; Optovue) and retinal imaging performed using Optomap 200° Ultra-Widefield (UWF) Digital retinal scan (Daytona) - Optos. The grading systems for DR used in this study were the National Screening Committee (NSC) classification and the International Clinical Diabetic Retinopathy Disease Severity Scale approved by American Academy of Ophthalmology [8]. The last blood samples for all subjects were reviewed. Readings of serum TC, LDL-C, HDL-C, TG, plasma glucose and glycated haemoglobin (HbA1c) were taken. LDL-C was calculated by Friedewald’s formula. In addition, body mass index (BMI) and 10- year risk of Atherosclerotic Cardiovascular Disease (ASCVD) were calculated. Additionally, measurements of blood pressure for all patients were reviewed. Hypertension was defined according to the current guidelines as BP levels ≥ 140/90 mmHg or the use of antihypertensive drugs [9].
The data were analysed using the statistical package for social sciences (SPSS) software (version 21). Data were presented as means ± standard deviation or percentages. Differences between the studied groups were examined using unpaired t-test or the Mann-Whitney U-test for parametric and non-parametric data, respectively, while a chi-square test was used for categorical data. 10-year risk of ASCVD was calculated using heart risk calculator [10]. Assessment of the relation between dyslipidemia and DR was performed using one-way analysis of variance (ANOVA) with Tukey-Kramer Multiple Comparisons Test. P-value < 0.05 was considered statistically significant.
Demographic and clinical laboratory characteristics of all participants in both groups are shown in (Table 1). Regarding the relation between DR and DM duration in this study, a high statistically significant difference was demonstrated between the 2 groups (P<0.0001) (Figure 1). The percentage of DR had slightly increased in women versus men (56.5% vs 43.5%), but without statistically significant difference (Figure 2). There was a statistically significant difference between the two groups relating to smoking (P<0.0001). There were 70% of smokers presented with DR compared to 30% of smokers without DR (Figure 3). Group 1 showed a statistically significant higher systolic and diastolic blood pressure than group 2 (P<0.0001) and (P=0.0289) respectively (Table 1, Figure 4).

Figure 1: Association between diabetic retinopathy (DR) and diabetes mellitus (DM) duration.
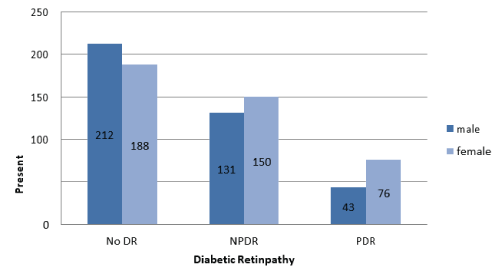
Figure 2: Association between diabetic retinopathy (DR) and sex.
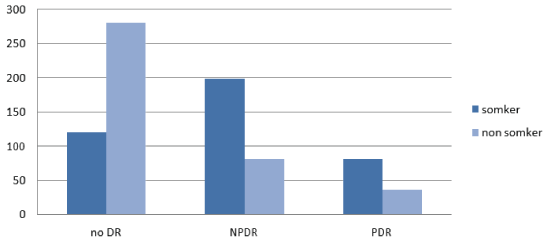
Figure 3: Association between diabetic retinopathy (DR) and smoking habit.
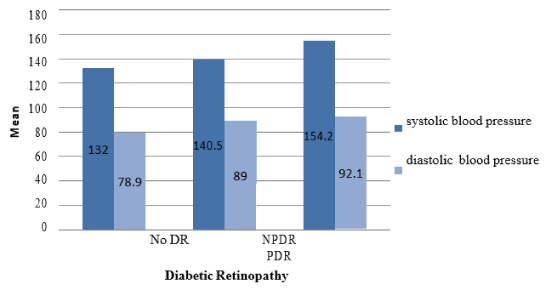
Figure 4: Association between diabetic retinopathy (DR) and blood pressure.
| Variable | With DR | Without DR | P (2 tailed) Unpaired t test |
| Male/Female (%) | 212 (53%)/188 (47%) | 197 (49.25%)/203 (50.75%) | |
| Mean age | 47.67 ± 6.43 | 46.30 ± 6.70 | 0.205 |
| Diabetic duration (year) | 21.69 ± 8.68 | 18.62±8.54 | < 0.0001* |
| Smokers (%) | 205 (51.25%) | 120(30%) | < 0.0001* |
| TC (mmol/L) | 5.34 ± 1.06 | 5.10 ± 1.03 | < 0.0001* |
| TG (mmol/L) | 3.26±1.91 | 3.26±1.65 | 0.2624 |
| HDL-C (mmol/L) | 1.24 ± 0.41 | 1.33 ± 0.44 | 0.0027* |
| LDL-C (mmol/L) | 3.05 ± 0.74 | 2.79 ± 0.78 | < 0.0001* |
| Hypertension (%) | 318 (79.5%) | 210 (52.5%) | < 0.0001* |
| Systolic blood pressure (mm Hg | 137.49 ± 17.58 | 131.98±21.18 | < 0.0001* |
| Diastolic blood pressure (mm Hg) |
74.35 ±13.39 | 72.30 ± 13.14 | 0.0289* |
| BMI (kg/m2) | 27.24 ± 5.55 | 28.68 ± 6.00 | 0.0005* |
| HbA1c | 10.06 ± 1.76 | 9.52 ± 3.79 | 0.0104* |
| ASCVD risk factor (%) | 56% | 32% | < 0.0001* |
Table 1: Demographic and clinical laboratory characteristics of all participants in both groups.
*P value <0.05 (*) was considered statistically significant.
Regarding assessment of dyslipidemia: Group 1 documented a statistically significant higher levels of TC, LDL-C and low levels of HDL-C (0.0013*, < 0.0001* and 0.0027*) respectively. But no statistically significant difference was detected between the 2 groups relating to TG (P=0.5478) (Table 2). This study documented a statistically significant association between the severity of DR and dyslipidemia, especially TC (P<0.001) and LDL-C level (P<0.003) (Table 3).
| Variable | DR | P value | ||
| Absent | NPDR | PDR | ||
| TC (mmol/L) | 5.10±1.03 | 5.25±1.05 | 5.60±1.07 | < 0.0001* |
| TG (mmol/L) | 3.26±1.65 | 3.86±1.91 | 1.86±0.82 | 0.5478 |
| HDL-C (mmol/L) | 1.33±0.44 | 1.24±0.44 | 1.20±0.44 | 0.0016* |
| LDL-C (mmol/L) | 2.79±0.78 | 2.99±0.74 | 3.14±0.72 | < 0.0001* |
| ASCDV risk factor (%) | 32% | 39% | 48% | 0.0318* |
Table 2: Association between dyslipidemia and diabetic retinopathy.
*P value <0.05 (*) was considered statistically significant.
| Grading of DR | Mild NPDR | Moderate NPDR | Severe NPDR | PDR | P value |
| TC(mmol/L) | 5.21 ± 1.09 | 5.29 ±1.10 | 5.41 ± 1.03 | 5.60 ± 1.07 | <0.001* |
| LDL-C level (mmol/L) | 2.79 ± 0.78 | 2.81 ± 0.89 | 2.87 ± 0.58 | 3.14 ± 0.72 | <0.003* |
| HDL-C level (mmol/L) | 1.26 ± 0.43 | 1.23 ± 0.32 | 1.21 ± 0.82 | 1.20 ± 0.44 | 0.102 |
| TG level (mmol/L) | 1.75 ±1.00 | 1.82 ± 0.63 | 1.83 ± 0.76 | 1.86 ± 0.82 | 0.361 |
Table 3: Association between dyslipidemia and severity of diabetic retinopathy.
P value <0.05 (*) was considered statistically significant.
Evaluation of the type of DR and presence of DME in this study: 64.05% of non-proliferative diabetic retinopathy (NPDR) patients had DME and 67.22% of proliferative diabetic retinopathy (PDR) patients had DME (Table 4). Table 5 shows number of patients according to the severity of DR and presence of DME. Patients with DME had a statistically significant high LDL-C and low HDL-C levels than patients without DME (P<0.002) and (P<0.003) respectively. But, no statistically significant differences were detected between the 2 groups concerning TG (P=0.180) and TC (P=0.151) (Table 6).
| Type of DR | DME+ | DME- | Total |
| NPDR | 180 (64.05%) | 101 | 281 |
| PDR | 80 (67.22%) | 39 | 119 |
Table 4: Number of patients according to the type of diabetic retinopathy and presence of diabetic macular edema (DME).
| Grade of DR | 0 | 1 | 1+DME | 2 | 2+DME | 3 | 3+DME | 4 | 4+DME | Total |
| Number of patients | 400 | 33 | 63 | 21 | 42 | 47 | 75 | 39 | 80 | 800 |
| Incidence % | 50 | 4.12 | 7.87 | 2.9 | 5.25 | 5.87 | 9 | 4.87 | 10 | 100 |
Table 5: Number of patients according to the severity of diabetic retinopathy (DR) and presence of diabetic macular edema (DME).
| DR with DME | DR without DME | P value | |
| TC level (mmol/L) |
5.21 ± 1.09 | 5.29 ±1.10 | 0.151 |
| LDL-C level (mmol/L) | 2.82 ± 0.78 | 2.71 ± 0.89 | <0.003* |
| HDL-C level (mmol/L) | 1.22 ± 0.43 | 1.63 ± 0.32 | <0.002* |
| TG level (mmol/L) | 1.75 ± 1.00 | 1.82 ± 0.63 | 0.18 |
Table 6: Assessment of dyslipidemia in diabetic retinopathy patients with & without diabetic macular edema.
*P value <0.05 (*) was considered statistically significant.
Regarding evaluation of 10-year risk of ASCVD: Group 1 showed a statistically significant higher ASCVD risk factor than group 2 (56%) and (32%) respectively (P<0.0001) (Table 2). Figure 5 demonstrated ASCVD risk factor in patients without DR (32%), with NPDR (39%), and with PDR (48%). There was a statistical significant difference between them (P<0.0318).
Figure 5: Atherosclerotic Cardiovascular Disease (ASCVD) risk factor in patients without diabetic retinopathy (DR) versus non-proliferative diabetic retinopathy (NPDR) patients and proliferative diabetic retinopathy (PDR) patients.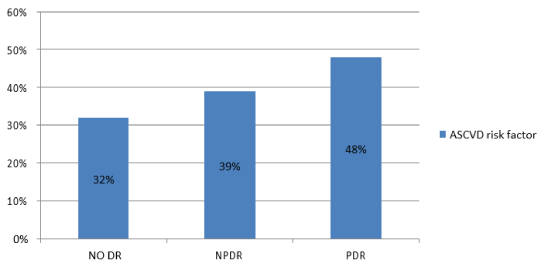
DR is demonstrated in more than 60% of type 2 DM patients during the first 20 years of the disease [11]. Many published articles found that poor glycemic control is markedly associated with DR [12]. However, some diabetic patients with modest hyperglycaemia show progressive DR, while others with highest hyperglycaemia over many years rarely demonstrate significant progression [13]. This may denote that DR pathogenesis may be attributed to other factors. There is a common link between DM and dyslipidemia. DR incidence depends on many factors such as the type and severity of diabetes, glycemic control, nutritional status, age and others [14].On the other hand, the relation between pathogenesis of DR and dyslipidemia remains unclear. It is supposed that dyslipidemia is a risk factor for its development. DME is the most common cause of visual loss in diabetic patients and can occur at any stage of DR. Also, the link between pathogenesis of DME and dyslipidemia is still vague.
This study evaluated the association between DR, dyslipidemia, DME, and blood pressure in subjects with type 2 DM. The results showed that patients with DR had significantly higher values of TC and LDL-C and lower HDL-C levels compared to patients without DR. Although TG was higher in patients with DR, there was no statistically significant difference between the 2 groups. Also, our findings demonstrated presence of association between dyslipidemia and the presence and severity of DR. Our results matched with Hu et al. [15] study that reported significant association between low apolipoprotein A1/apolipoprotein B ratio in serum and PDR in type 2 diabetic patients of over 15 years’ duration. In addition, Gadi and Samaha et al. [16] study demonstrated a strong prevalence of lipid abnormalities in patients with DR. Moreover, our findings agreed with Timothy et al. [17] study that documented positive association between the severity of retinopathy and TG level and negative association with HDL-C. Additionally, our findings matched with Ganeswaran et al. [18] study that showed significant association between DR and high levels of TC and LDL-C. On the other hand, the United Kingdom Prospective Diabetes Study (UKPDS) results documented that higher HDL-C level was associated with more severe DR. But, the reason of this association was not identified. However, the severity of DR was not related to TG level and LDL-C level [19].Also, the Multi-Ethnic Study of Atherosclerosis (MESA) [20] evaluated the risk factors for DR in a multi-ethnic US population of whites, blacks, Hispanics, and Chinese and found no associations of plasma lipids and either retinopathy or macular edema in 778 individuals aged from 45 to 85 years with DM.
Our study showed a statistically significant difference between the 2 groups relating to DM duration. Regarding the sex, no statistically significant difference between the 2 groups was detected. In addition, we found a statistically significant difference between the 2 groups as regards to smoking. Additionally, level of systolic and diastolic blood pressure between the 2 groups showed a statistically significant difference. Nearly all participants in our study were overweight or obese with BMI ≥ 25 Kg/m2.
Our findings concerning duration, sex, smoking, and blood pressure agreed with several studies such as, Al Aldebasi et al. [21] and El Mofty et al. [22] studies that documented a statistically significant relationship between DR and dyslipidemia and all these variables. Furthermore, the Hoorn Study [23] demonstrated positive association of DR with systemic hypertension, BMI, serum cholesterol and TG level. In addition, systemic hypertension and plasma TC and LDL-C levels found correlations with retinal hard exudates. Dyslipidemia in diabetics may be explained by specific abnormalities in lipoprotein metabolism and/or abnormalities in insulin action [23].
Concerning assessment of ASCVD risk factor, the current study showed a statistically significant difference between the 2 groups (56%) and (32%) respectively (P<0.0001) This finding matched with the Cardiovascular Health Study (CHS) which confirmed positive association of DR with higher average systolic blood pressure, higher TC and LDL-C levels, and the presence of cardiovascular disease [24].
Regarding relationship between dyslipidemia and DME in the current study, patients with DME have high LDL-C level and low HDL-C level than patients without DME. However, no statistically significant differences regarding TC and TG between the two groups were reported. These results agreed with the Hoorn [23] and Suwal et al. [25]studies that confirmed positive association between retinal hard exudates and high serum LDL-C level. Also, the Early Treatment Diabetic Retinopathy Study (ETDRS) [26] documented correlation between elevated TG and LDL-C and high risk of exudative maculopathy. Additionally, Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) XIII [27] demonstrated a significant association between increasing severity of DR and of retinal hard exudates and high TC in insulin-dependent diabetics. Likewise, Golubovic study [28] found significantly high levels of TG, TC, and cholesterol ester in type 2 DM patients with DME compared to those without DME. But, there were no statistically significant differences of HDL-C and LDL-C levels between the 2 groups although higher values were detected in patients with DME. Moreover, Sachdev and Sahni study [29] in North India documented significant correlation between retinal hard exudates and systolic blood pressure, TC, LDL-C, and TG levels.
The differences between all the studies which might affect the results may be caused by many factors such as, sex, age, duration, race, and sample size of included subjects in each study.
This study documented significant association between DR and dyslipidemia in type 2 diabetic patients. In addition, the study confirmed that patients with DME had significant high LDL-C level and low HDL-C level than patients without DME. Moreover, the current study demonstrated significant association between DR and hypertension. Therefore, efficient control of hyperglycemia, dyslipidemia and hypertension would be of great value in delaying the progression of DR in these patients.
Ethics approval and consent to participate: The study got the approval of Research Ethics Committee of College of Applied Medical Sciences, King Saud University and Research Ethics Committee in Prince Sultan Military Medical City, Riyadh, Saudi Arabia. All procedures performed in this study were in accordance with Helsinki declaration and its later amendments. All the participants signed comprehensive consent after explanation of the possible consequences of the study prior to investigations.
not applicable
not applicable
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers› bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patentlicensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
No funding was received for this research
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Elagamy A, Al Enazy BR, AL Zaaidi SE (2018) Association between Dyslipidemia and Diabetic Retinopathy in Type 2 Diabetic Patients. J Ophthalmic Stud 1(1): dx.doi.org/10.16966/2639-152X.111
Copyright: © 2018 Elagamy A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access