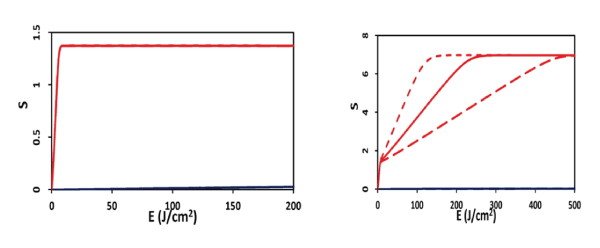
Figure 1: Efficacy versus light fluence (or dose) for the case of p=0 (left figure), and p=0.07 (uM/s).


Jui-Teng Lin*
New Vision Inc, Taipei, Taiwan*Corresponding author: Jui-Teng Lin, New Vision Inc, Taipei, Taiwan, E-mail: jtlin55@gmail.com
Photochemical kinetics for the efficacy of PDT is analyzed to show the critical factors of efficacy including: the concentrations of photosensitizers and oxygen in the treated target, the exposure time, intensity and does (energy) of the light applied to the target. Higher light intensity has faster rising curve of the efficacy, but it reaches the same steady-state value as that of low intensity. Higher initial concentration of oxygen and photosensitizers, C0 , always provide higher efficacy. Minimum light dose and/or less exposure time for accelerated procedure by using a higher intensity (but same dose, E0 ) are desired. Threshold product of drug-light dose [C0E0 ] * is derived showing that larger C0 has a lower E0* and vice versa. However, higher intensity requires higher threshold energy, and does not follow the Bunsen-Roscoe law (BRL) of reciprocity, when there is an oxygen source term.
Photodynamic therapy; Photosensitizers; Reactive oxygen species; Threshold dose; Verteporfin; Age related macular degeneration; Modeling
Photodynamic therapy (PDT) is a photochemical process has been used in many industrial (polymer) and medical applications. PDT was first introduced to ophthalmology in 2000, is a therapeutic procedure which utilizes the photosensitive intravenous drug, verteporfin (Visudyne, Bausch and Lomb) in combination with a low power, long duration infrared laser to treat vascular issues in the retina and choroid [1,2]. It was first indicated for neovascular age related macular degeneration (AMD), with large randomized clinical trials showed an improvement in visual acuity versus placebo [3]. The studies proved PDT’s efficacy in treating AMD patients with classical subfoveal choroidal neovascularization. PDT is now most often used to treat Central Serous Retinopathy (CSR) and have been shown to be effective by several published studies [3].
Indocyanine green angiography (ICGA)-guided photodynamic therapy (PDT) with verteporfin (Visudyne; Novartis AG, Bulach, Switzerland) has been shown to be effective in treating chronic CSR with both good anatomical and visual function [3]. However, the use of the standard full energy protocol of PDT has been reported to be associated with adverse effects, such as transient reduction of macular function, RPE atrophy, choroidal no perfusion or ischemia, and secondary choroidal neovascularization [3]. Reduction of the total light energy (dose) may reduce damage to the underlying normal choroidal vasculature and RPE. It was r revealed that half- dose PDT was effective not only in improving the visual outcomes but also in avoiding recurrence rate in a long-term study [4-8].
There are 2 methods used to achieve this objective:
Effort to minimize these complications, various modified PDT protocols have been explored involving reduced verteporfin dosage, laser fluence, or a combination of both. Half-fluence, half-dose, half-fluence and micropulse, 1/3 dose, and minimal-fluence protocols have all demonstrated some degree of treatment effect. Half-dose verteporfin and half-fluence treatments are the two most described modified protocols [4-8].
Verteporfin was the second generation photosensitizer developed to treat wet AMD with an intensified longwavelength absorption maxima at approximately 690 nm, compared to the first generation photosensitizer Photofrin with a weaker wavelength absorption (630 nm), indicating a 50% increase in tissue penetration by light [1]. Moreover, Verteporfin can be rapidly cleared from the body, minimizing patient photosensitivity to 1 to 2 days. Verteporfin has been approved for the treatment of wet AMD since 1999 by the USFDA.
Factors influencing the efficacy of PDT include: selectivity, penetration and optimization, where maximum light penetration depth and efficacy, minimum dose (or treatment time), and high selectivity are desired. However, minimum treatment time and maximum therapy efficacy are two competing factors and cannot be easily overcome. Optimal combination of light energy (dose), intensity and irradiation time may be achieved via Lin-scaling laws, Arndt-SchulzLaw (for therapeutic window) and Bunsen-Roscoe law (for reciprocity rule) [9-13]. Bunsen-Roscoe law (BRL) of reciprocity stating that the effect of a photo-biological reaction is proportional only to the total irradiation fluence (or light dose) (E=It), or the product of intensity (I) and exposure time (t). To achieve the same efficacy, the required exposure time based on BRL is given by t=E/I. Based on BRL, treatment time may be shortened by using a higher intensity while maintaining the similar efficacy. However, BRL is still controversial and has limited validation, as reported by Lin [13].
Oxygen plays a critical role in the efficacy of Type-II PDT [14-17], where oxygen consumption and diffusion effects in PDT was first reported by Foster et al [15] in 1991 and was updated and reviewed recently by Zhu at al [16,17] in 2017. The kinetics of both oxygen-mediated (type-II) and non-oxygenmediated (type-I) was reported by Lin recently [14]. For ophthalmic applications, the reciprocity law was reported for the role of drug-light dose on the PDT efficacy [13,14].
The role of oxygen in Type-II PDT has been studied in corneal cross linking [14] and phototherapy of various cancers [15-17]. However, it has not been explored for AMD.
In this study, we will analyze the efficacy of PDT. Minimum light dose and/or less exposure time for accelerated procedure by using a higher intensity (but same dose, E0) are desired. We will show the nonlinear feature of drug-light dose. We will show that a threshold product of [C0E0]* and larger C0 has a lower threshold energy E0* . However, higher intensity requires higher threshold energy and does not follow the Bunsen-Roscoe law (BRL) of reciprocity [9] when there is an oxygen source term. This non-BRL is a new finding in this study and will provide useful clinical guidance for fast and effective PDT.
PDT with verteporfin causes release of free radicals when the verteporfin is activated by the laser energy. The reaction that ensues between the free radicals and blood vessel endothelial cell membranes cause locally increased histamines, thromboxane and TNF-α, all immune modulation factors. The antiinflammatory response can lead to series of events including vasoconstriction, thrombosis, increased vascular permeability, blood stasis and hypoxia [1]. In the case of neovascularization, this process serves to induce regression of these harmful blood vessels. After injected into the bloodstream, the Visudyne (6 mg/m2 dose) selectively collects in the abnormal blood vessels in the retina and choroid. Fifteen minutes after intravenous infusion, low power laser is applied (standard dose of 50 J/cm2 , irradiance of 600 mW/cm2 of 689 nm light over 83 seconds) which activates the phototoxic Visudyne to seal leaking blood vessels by generating these free radicals in areas of necessary treatment [3].
PDT makes use of photosensitizers (PS) to generate reactive reactive species upon the absorption of specific wavelengths of light, where the selectivity is given by: (i) PSs are preferentially taken up by tumour tissues, and (ii) the molecules generate cytotoxic radical species only at the site where light is administered. There are two cytotoxic photochemical mechanisms in PDT (Figure 1): (i) Type-I mechanism where the molecule directly reacts through its triplet excited state to generate reactive radicals species; and (ii) Type –II mechanism where PSs convert molecular oxygen into highly reactive singlet oxygen. Most PSs currently used in the clinic are predominantly oxygen-mediated Type –II molecules. It is also possible that both Type-I and –II coexist.

Figure 1: Efficacy versus light fluence (or dose) for the case of p=0 (left figure), and p=0.07 (uM/s).
Most PS available for PDT utilizes Type II photodynamic processes, i.e., the photodynamic effect is achieved through the production of singlet oxygen [8,13]. (Figure 1) the process begins with the absorption of a photon by PS in its ground state, promoting it to an excited state. The PS molecule can return to its ground state by emission of a fluorescence photon, or convert to a triplet state which may undergo a collisional energy transfer with ground state molecular oxygen (type II process), or with the substrate/target (type I process). In type II interaction, the PS returns to its ground state, and oxygen is promoted from its ground state (a triplet state) to its excited (singlet) state. In type-II process, the PS is almost not consumed (due to the slow singlet oxygen quenching rate), whereas in type-I process the PS is largely depleted specially for high intensity [8].
Quasi-steady state macroscopic kinetic equation for the concentration of the ground state PS, C (z, t), the ground state oxygen, [O2], and the light intensity, I (z, t), are given by [14-17].
\[\frac{{\partial C\left( {z,t} \right)}}{{\partial t{\rm{ }}}}{\rm{ }} = - b\left( {g\left[ A \right] + g'} \right)C{\rm{ }}\,\,\,\,\,\,\,\,\,\left( {1.{\rm{a}}} \right)\]
\[\frac{{\partial \left( {{O_2}} \right)}}{{\partial t{\rm{ }}}}{\rm{ }} = - bG + P{\rm{ }}\,\,\,\,\,\,\,\,\,\left( {1.{\rm{b}}} \right){\rm{ }}\]
\[\frac{{\partial I\left( {z,t} \right)}}{{\partial {\rm{ }}}}{\rm{ }} = - A'\left( {z,t} \right)I\left( {z,t} \right)\,\,\,\,\,\,\,\,\,{\rm{ }}\left( {1.{\rm{c}}} \right)\]
where b=aqI (z, t); g=(k8 /k3 ) G0 ; g’=K12(C+d’) [O2 ] G0 , G=C[O2 ] G0; G0 =1([O2 ] +k+L), k=k5 /k3; L=(k8 /k3 ) [A]; K12= (s1 k11/K1 + s2 k12/K2 ); K1 = k11(C+d’) +k71[A]; K2 = k6 +k12(C+d’) +k72[A]; a is a coupling constant; q is the triplet state [T] quantum yield given by q=k2 /(k1 +k2 ); s2 and s1 are the fraction of [O2] converted to the singlet oxygen and other ROS, respectively, in type-II and type-I; d’ is a low concentration correction to count for the limited diffusion distance of the reactive species [13]. We have also included in Eq. (1.b) the oxygen source term P (z, t) =p(1-[O2]/ [O2]0), with a rate constant p to count for the situation when there is an external continuing supply, or nature replenishment (at a rate of p), besides the initial oxygen in the treated tissue.
Eg. (1.c) defines the dynamic light intensity including the effect due to depletion of C (z, t) due to light intensity, with an effective absorption coefficient given by A’ (z, t) =a’C (z, t).
In the quasi-steady state, the singlet oxygen concentration of the reactive oxygen species (ROS) are given by: [O’] =bs1K1G, for the superoxide anion; and [1O2 ]=bs2 K2 G, for the singlet oxygen. [O’] and [1O2 ] represent, respectively, the amounts of reactive oxygen species (ROS) in type-I and type-II mechanism. Moreover, PDT efficacy is given by Eff=1-exp(- S1+S2), with S1 and S2 are the S-function (for type-I and typeII) given by the time-integral of bgC and bG, respectively, for type-I and type-II.
\[S1 = f\smallint _0^t\sqrt {bgC(z,t)dt{\rm{ }}} \]
\[S2 = f\smallint _0^tbg'Gdt{\rm{ }}\,\,\left( {2.{\rm{b}}} \right)\]
Where, f is the fraction of ROS interacting with [A]. Here, S2 relates to the fraction of acceptors that reacted due to (ROS)- mediated reactions, and S1 relates to the fraction that reacts under hypoxic conditions or any other non-oxygen-mediated reactions, such as triplet interactions.
For type-II dominant case, with g<<g’, the first-order solution of Eq. (1.a) gives C (z, t) =C0exp(-b’t), with b’=bKd’, which is used to solve for Eq. (1.b), the oxygen concentration [O2]=X (z, t). For the small b’<0.0001, C (z, t) is a slowly depletion function comparing to the oxygen, one may assume a constant C (z, t)=C0 to find the approximated X and S2 as follows. X+klnX=X00 – BtC0 , with B=b2+b3 t, b2 =b+(1-X’) P, b3 =bC0 X’P; X’=X00/X0 ; X00= X0 +klinX0. The type-II S-function, S2 , is given by the time integral of the singlet oxygen, or the integral of, eq. (2.b), given by S2 =aqEC0 [1-(k/X00) (1+0.5b2 t+0.33b3 t2 )], which is proportional to the product of C0X0E0 with E0 =tI0 being the light fluence (dose). However, it has a nonlinear saturation due to the nonlinear term NL=(k/X00) (1+0.5b2 t+0.33b3 t2).
The S2 formula provides us the following important features (Figure 2, for the case of no oxygen source terms, with P=0): (a) for transient state, with bt<<1, S2 (at z=0) = aqEC0 [1-(k/X00)], so that S2 is proportional to the product of C0 E0 , and follows the linear Bunsen-Roscoe law (BRL) of reciprocity; (b) for large time, S2 is a nonlinear function of C0E0 , and is given by S2 =aqY [a1 -a2 Y- a3 Y2 ], with Y= C0 E0 . The transient S also defines the threshold of cumulated singlet oxygen concentration, defined by when CV<0.36, or S2 >S2* =1.0, which defines the threshold product of aqEC0 [1-(k/X00)] =1.0, or [C0E0]* > [1+(k/X00)]/(aq). Therefore, larger C0 and/or X0 , has a lower E0* and vice versa. (Figure 1) shows the efficacy (S2) versus light fluence (or dose) for the case of p=0 and p=0.07 (uM/s).
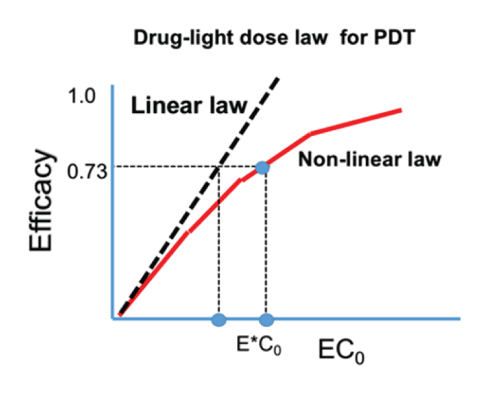
Figure 2: The efficacy versus product [EC0 ] showing the linear Bunsen-Roscoe law (BRL, dashed curve) and nonlinear law (solid curve). Also shows are the threshold products.
Based on (Figure1 and 2) the important features of PDT efficacy and the drug-light dose law are summarized as follows:
(a) High intensity depletes [3O2] and C (z, t) faster such that it produces high singlet oxygen [1O2] and has high efficacy, in the transient state. However, they reach the same steady-state efficacy (or S2 function) as that of low intensity.
(b) As shown by Figure 2, (for the case of P=0), the linear BRL underestimates the threshold product [EC0]* comparing to the nonlinear BRL. In addition, the previous reported cases of: half-dose with full fluence; full-fluence with half dose; and 1/3 dose with 3times-fluence; all have the same product of [EC0]. However, the lower-fluence case should be the optimal (with highest efficacy, if it is above the efficacy threshold.
(c) High concentration (C0) provides large efficacy, however, it has shallow PDT depth, for the same dose. Therefore, optimal C0 is required depending on conditions of AMD.
(d) For P=0, low and high intensity reach the same efficacy, when they have the same dose (Figure 2). Under the BRL, the S-E curves overlap for various intensity (with the same dose, and under the condition that oxygen source term P=0). However, for P>0, BRL does not valid and high intensity which may accelerate the procedure, however, longer exposure time (or dose) is needed than what is predicted by the BRL, t=E/I0.
(e) As shown by Figure 1, for the same dose (with P>0), low intensity is always more efficient in the transient state, although all intensities reach the same steady state efficacy. This new finding was not reported previously.
The role of oxygen in Type-II PDT has been studied in corneal cross linking [14] and phototherapy of various cancers [15-17]. Clinically, similar to cancer therapy, oxygen can be continuously supplied by the blood circulation to the targeted area of AMD. However, it might take time to compensate the oxygen level depleted by the red-light. Oxygen-enhanced corneal cross linking [14] can be achieved under a highpressured oxygen goggle attached to the eye. However, it is hard to do it in cancer or AMD, where the oxygen supply could only via blood circulation. The features analyzed in this study, non-BRL and drug-light dose law, can be generalized to other PDT having Type-II is the dominant processes such as cancer therapy, which was presented elsewhere [18].
To achieve the same efficacy, minimum dose and/or less exposure time for accelerated procedure by using a higher intensity (but same dose) is highly needed. However, higher intensity requires higher threshold energy and it does not follow the BRL reciprocity law, when there is an oxygen source term.
Lin is the CEO of New Vision Inc. and has financial interest.
This work was supported by the internal grant of New Vision Inc.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Lin JT (2018) Analysis of Drug-Light Dose on the Efficacy of Photodynamic Therapy of Age Related Macular Degeneration. J Ophthalmic Stud 1(1): dx.doi.org/10.16966/2639-152X.108
Copyright: © 2018 Lin JT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access